Preparation and Application of a Novel S-Scheme Nanoheterojunction Photocatalyst (LaNi0.6Fe0.4O3/g-C3N4)
- PMID: 38973884
- PMCID: PMC11223155
- DOI: 10.1021/acsomega.4c02333
Preparation and Application of a Novel S-Scheme Nanoheterojunction Photocatalyst (LaNi0.6Fe0.4O3/g-C3N4)
Abstract
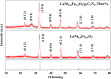
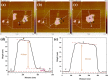
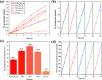
Rapid recombination of photogenerated electrons and holes affects the performance of a semiconductor device and limits the efficiency of photocatalytic water splitting for hydrogen production. The use of an S-scheme nanoscale heterojunction catalyst for the separation of photogenerated charge carriers is a feasible approach to achieve high-efficiency photocatalytic hydrogen evolution. Therefore, we synthesized a three-dimensional S-scheme nanoscale heterojunction catalyst (LaNi0.6Fe0.4O3/g-C3N4) and investigated its activity in photocatalytic water splitting. An analysis of the band structure (XPS, UPS, and Mott-Schottky) indicated effective interfacial charge transfer in an S-scheme nanoscale heterojunction composed of two n-type semiconductors. X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR) spectroscopy confirmed that the light-induced charge transfer followed the S-scheme mechanism. Based on the capture test (EPR) of •OH free radicals, it can be seen that the enhanced activity is attributed to the S-scheme carrier migration mechanism in heterojunction, which promotes the rapid adsorption of H+ by the abundant amino sites in g-C3N4, thus effectively generating H2. The 2D/2D LaNi0.6Fe0.4O3/g-C3N4 heterojunction has a good interface and produces a built-in electric field, improving the separation of e- and h+ while increasing the oxygen vacancy. The synergistic effect of the heterostructure and oxygen vacancy makes the photocatalyst significantly better than LaNi0.6Fe0.4O3 and g-C3N4 in visible light. The hydrogen evolution rate of the composite catalyst (LaNi0.6Fe0.4O3/g-C3N4-70 wt %) was 34.50 mmol·h-1·g-1, which was 40.6 times and 9.2 times higher than that of the catalysts (LaNiO3 and g-C3N4), respectively. After 25 h of cyclic testing, the catalyst (LaNi0.6Fe0.4O3/g-C3N4-70 wt %) composite material still exhibited excellent hydrogen evolution performance and photostability. It was confirmed that the synergistic effect between abundant active sites, enriched oxygen vacancies, and 2D/2D heterojunctions improved the photoinduced carrier separation and the light absorption efficiency of visible light. This study opens up new possibilities for the logical design of efficient photodecomposition using 2D/2D heterojunctions combined with oxygen vacancies.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Dai M.; He Z.; Zhang P.; Li X.; Wang S. ZnWo4-ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. 10.1016/j.jmst.2022.02.014. - DOI
-
- Xi Y.; Chen W.; Dong W.; Fan Z.; Wang K.; Shen Y.; Tu G.; Zhong S.; Bai S. Engineering an interfacial facet of S-scheme heterojunction for improved photocatalytic hydrogen evolution by modulating the internal electric field. ACS Appl. Mater. Interfaces 2021, 13 (33), 39491–39500. 10.1021/acsami.1c11233. - DOI - PubMed
LinkOut - more resources
Full Text Sources
