The APPLE trial in the evolving landscape of ctDNA monitoring
- PMID: 38973953
- PMCID: PMC11225046
- DOI: 10.21037/tlcr-24-185
The APPLE trial in the evolving landscape of ctDNA monitoring
Keywords: EGFR mutations; T790M; circulating tumor DNA (ctDNA); switch to osimertinib.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-185/coif). M.N. is a member of these advisory boards: AstraZeneca, Daiichi Sankyo, Takeda, Novartis, EMD Serono, Janssen, Pfizer, Eli Lilly and Company, Bayer, Regeneron, BMS, and Genentech; a consultant at Caris Life Sciences (virtual tumor board); a speaker at Blueprint Medicines, Janssen, Mirati and Takeda; and reports travel support from AnHeart Therapeutics and stock/stock options from MBrace Therapeutics. The other author has no conflicts of interest to declare.
Figures
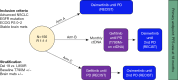
Comment on
-
Osimertinib treatment based on plasma T790M monitoring in patients with EGFR-mutant non-small-cell lung cancer (NSCLC): EORTC Lung Cancer Group 1613 APPLE phase II randomized clinical trial.Ann Oncol. 2023 May;34(5):468-476. doi: 10.1016/j.annonc.2023.02.012. Epub 2023 Feb 28. Ann Oncol. 2023. PMID: 36863484 Clinical Trial.
References
-
- Mok T, Wu YL, Lee JS, et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res 2015;21:3196-203. 10.1158/1078-0432.CCR-14-2594 - DOI - PubMed
-
- Sorensen BS, Wu L, Wei W, et al. Monitoring of epidermal growth factor receptor tyrosine kinase inhibitor-sensitizing and resistance mutations in the plasma DNA of patients with advanced non-small cell lung cancer during treatment with erlotinib. Cancer 2014;120:3896-901. 10.1002/cncr.28964 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
