Poly(p-coumaric acid) nanoparticles alleviate temporomandibular joint osteoarthritis by inhibiting chondrocyte ferroptosis
- PMID: 38973989
- PMCID: PMC11224931
- DOI: 10.1016/j.bioactmat.2024.06.007
Poly(p-coumaric acid) nanoparticles alleviate temporomandibular joint osteoarthritis by inhibiting chondrocyte ferroptosis
Abstract
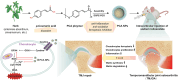
Oxidative stress and inflammation are key drivers of osteoarthritis (OA) pathogenesis and disease progression. Herein we report the synthesis of poly(p-coumaric) nanoparticles (PCA NPs) from p-courmaic acid (p-CA), a naturally occurring phytophenolic acid, to be a multifunctional and drug-free therapeutic for temporomandibular joint osteoarthritis (TMJOA). Compared to hyaluronic acid (HA) that is clinically given as viscosupplementation, PCA NPs exhibited long-term efficacy, superior anti-oxidant and anti-inflammatory properties in alleviating TMJOA and repairing the TMJ cartilage and subchondral bone in a rat model of TMJOA. Notably, TMJ repair mediated by PCA NPs could be attributed to their anti-oxidant and anti-inflammatory properties in enhancing cell proliferation and matrix synthesis, while reducing inflammation, oxidative stress, matrix degradation, and chondrocyte ferroptosis. Overall, our study demonstrates a multifunctional nanoparticle, synthesized from natural p-coumaric acid, that is stable and possess potent antioxidant, anti-inflammatory properties and ferroptosis inhibition, beneficial for treatment of TMJOA.
Keywords: Cartilage; Ferroptosis; Oxidative pressure; Poly(p-coumaric acid) nanoparticles; Temporomandibular joint osteoarthritis.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Quicke J.G., Conaghan P.G., Corp N., Peat G. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthritis Cartilage. 2022;30(2):196–206. - PubMed
-
- Wang X.D., Zhang J.N., Gan Y.H., Zhou Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015;94(5):666–673. - PubMed
-
- Barry F., Chai F., Chijcheapaza-Flores H., Garcia-Fernandez M.J., Blanchemain N., Nicot R. Systematic review of studies on drug-delivery systems for management of temporomandibular-joint osteoarthritis. J Stomatol Oral Maxillofac Surg. 2022;123(5):e336–e341. - PubMed
LinkOut - more resources
Full Text Sources

