Transportation engineering for enhanced production of plant natural products in microbial cell factories
- PMID: 38974023
- PMCID: PMC11224930
- DOI: 10.1016/j.synbio.2024.05.014
Transportation engineering for enhanced production of plant natural products in microbial cell factories
Abstract
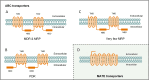
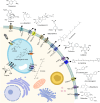
Plant natural products (PNPs) exhibit a wide range of biological activities and have essential applications in various fields such as medicine, agriculture, and flavors. Given their natural limitations, the production of high-value PNPs using microbial cell factories has become an effective alternative in recent years. However, host metabolic burden caused by its massive accumulation has become one of the main challenges for efficient PNP production. Therefore, it is necessary to strengthen the transmembrane transport process of PNPs. This review introduces the discovery and mining of PNP transporters to directly mediate PNP transmembrane transportation both intracellularly and extracellularly. In addition to transporter engineering, this review also summarizes several auxiliary strategies (such as small molecules, environmental changes, and vesicles assisted transport) for strengthening PNP transportation. Finally, this review is concluded with the applications and future perspectives of transportation engineering in the construction and optimization of PNP microbial cell factories.
Keywords: Microbial cell factories; Plant natural products; Transportation engineering; Transporters.
© 2024 The Authors.
Conflict of interest statement
None.
Figures


Similar articles
-
Engineering microbial cell factories for the production of plant natural products: from design principles to industrial-scale production.Microb Cell Fact. 2017 Jul 19;16(1):125. doi: 10.1186/s12934-017-0732-7. Microb Cell Fact. 2017. PMID: 28724386 Free PMC article. Review.
-
Reconstitution and optimization of complex plant natural product biosynthetic pathways in microbial expression systems.Curr Opin Biotechnol. 2024 Jun;87:103136. doi: 10.1016/j.copbio.2024.103136. Epub 2024 May 4. Curr Opin Biotechnol. 2024. PMID: 38705090 Review.
-
Transporter Engineering for Microbial Manufacturing.Biotechnol J. 2020 Sep;15(9):e1900494. doi: 10.1002/biot.201900494. Epub 2020 May 29. Biotechnol J. 2020. PMID: 32298528 Review.
-
Application of Metabolite-Responsive Biosensors for Plant Natural Products Biosynthesis.Biosensors (Basel). 2023 Jun 7;13(6):633. doi: 10.3390/bios13060633. Biosensors (Basel). 2023. PMID: 37366998 Free PMC article. Review.
-
Microbial Cell Factory for Efficiently Synthesizing Plant Natural Products via Optimizing the Location and Adaptation of Pathway on Genome Scale.Front Bioeng Biotechnol. 2020 Aug 14;8:969. doi: 10.3389/fbioe.2020.00969. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32923436 Free PMC article. Review.
Cited by
-
Efficient biodegradation and upcycling of polyethylene terephthalate mediated by cell-factories.Front Microbiol. 2025 Jul 2;16:1599470. doi: 10.3389/fmicb.2025.1599470. eCollection 2025. Front Microbiol. 2025. PMID: 40673144 Free PMC article. Review.
-
Enhanced chlorogenic acid production from glucose via systematic metabolic engineering of Saccharomyces cerevisiae.Synth Syst Biotechnol. 2025 Mar 20;10(3):707-718. doi: 10.1016/j.synbio.2025.03.004. eCollection 2025 Sep. Synth Syst Biotechnol. 2025. PMID: 40248482 Free PMC article.
References
-
- Paddon C.J., Westfall P.J., Pitera D.J., Benjamin K., Fisher K., McPhee D., Leavell M.D., Tai A., Main A., Eng D., Polichuk D.R., Teoh K.H., Reed D.W., Treynor T., Lenihan J., Fleck M., Bajad S., Dang G., Dengrove D., Diola D., Dorin G., Ellens K.W., Fickes S., Galazzo J., Gaucher S.P., Geistlinger T., Henry R., Hepp M., Horning T., Iqbal T., Jiang H., Kizer L., Lieu B., Melis D., Moss N., Regentin R., Secrest S., Tsuruta H., Vazquez R., Westblade L.F., Xu L., Yu M., Zhang Y., Zhao L., Lievense J., Covello P.S., Keasling J.D., Reiling K.K., Renninger N.S., Newman J.D. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496(7446):528–532. doi: 10.1038/nature12051. - DOI - PubMed
-
- Gao J.C., Zuo Y.M., Xiao F., Wang Y.L., Li D.F., Xu J.H., Ye C.F., Feng L.J., Jiang L.J., Liu T.F., Gao D., Ma B., Huang L., Xu Z.A., Lian J.Z. Biosynthesis of catharanthine in engineered Pichia pastoris. Nat. Synth. 2023;2(3):231–242. doi: 10.1038/s44160-022-00205-2. - DOI
-
- Wriessnegger T., Augustin P., Engleder M., Leitner E., Müller M., Kaluzna I., Schürmann M., Mink D., Zellnig G., Schwab H., Pichler H. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris. Metab Eng. 2014;24:18–29. doi: 10.1016/j.ymben.2014.04.001. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

