Diversity of genotypes and pathogenicity of H9N2 avian influenza virus derived from wild bird and domestic poultry
- PMID: 38974026
- PMCID: PMC11225357
- DOI: 10.3389/fmicb.2024.1402235
Diversity of genotypes and pathogenicity of H9N2 avian influenza virus derived from wild bird and domestic poultry
Abstract
Introduction: The H9N2 subtype is a predominant avian influenza virus (AIV) circulating in Chinese poultry, forming various genotypes (A-W) based on gene segment origins. This study aims to investigate the genotypic distribution and pathogenic characteristics of H9N2 isolates from wild birds and domestic poultry in Yunnan Province, China.
Methods: Eleven H9N2 strains were isolated from fecal samples of overwintering wild birds and proximate domestic poultry in Yunnan, including four from common cranes (Grus grus), two from bar-headed geese (Anser indicus), and five from domestic poultry (Gallus gallus). Phylogenetic analysis was conducted to determine the genotypes, and representative strains were inoculated into Yunnan mallard ducks to assess pathogenicity.
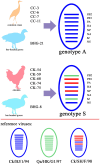
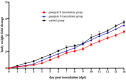
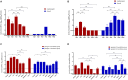
Results: Phylogenetic analysis revealed that five isolates from domestic birds and one from a bar-headed goose belong to genotype S, while the remaining five isolates from wild birds belong to genotype A. These bird-derived strains possess deletions in the stalk domain of NA protein and the N166D mutation of HA protein, typical of poultry strains. Genotype S H9N2 demonstrated oropharyngeal shedding, while genotype A H9N2 exhibited cloacal shedding and high viral loads in the duodenum. Both strains caused significant pathological injuries, with genotype S inducing more severe damage to the thymus and spleen, while genotype A caused duodenal muscle layer rupture.
Discussion: These findings suggest that at least two genotypes of H9N2 are currently circulating in Yunnan, and Yunnan mallard ducks potentially act as intermediaries in interspecies transmission. These insights highlight the importance of analyzing the current epidemiological transmission characteristics of H9N2 among wild and domestic birds in China.
Keywords: H9N2 AIV; bar-headed goose; common crane; genotype A; genotype S; pathologic analysis; phylogenetic analysis.
Copyright © 2024 Yang, Ji, Yang, Zhang, Yin, Dai, Wang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fereidouni S. R., Starick E., Grund C., Globig A., Mettenleiter T. C., Beer M., et al. (2009). Rapid molecular subtyping by reverse transcription polymerase chain reaction of the neuraminidase gene of avian influenza A viruses. Vet. Microbiol. 135, 253–260. 10.1016/j.vetmic.2008.09.077 - DOI - PubMed
LinkOut - more resources
Full Text Sources

