Hemospray® (hemostatic powder TC-325) as monotherapy for acute gastrointestinal bleeding: a multicenter prospective study
- PMID: 38974074
- PMCID: PMC11226744
- DOI: 10.20524/aog.2024.0897
Hemospray® (hemostatic powder TC-325) as monotherapy for acute gastrointestinal bleeding: a multicenter prospective study
Abstract
Background: Hemostatic powders are used as second-line treatment in acute gastrointestinal (GI) bleeding (AGIB). Increasing evidence supports the use of TC-325 as monotherapy in specific scenarios. This prospective, multicenter study evaluated the performance of TC-325 as monotherapy for AGIB.
Methods: Eighteen centers across Europe and USA contributed to a registry between 2016 and 2022. Adults with AGIB were eligible, unless TC-325 was part of combined hemostasis. The primary endpoint was immediate hemostasis. Secondary outcomes were rebleeding and mortality. Associations with risk factors were investigated (statistical significance at P≤0.05).
Results: One hundred ninety patients were included (age 51-81 years, male: female 2:1), with peptic ulcer (n=48), upper GI malignancy (n=79), post-endoscopic treatment hemorrhage (n=37), and lower GI lesions (n=26). The primary outcome was recorded in 96.3% (95% confidence interval [CI]: 92.6-98.5) with rebleeding in 17.4% (95%CI 11.9-24.1); 9.9% (95%CI 5.8-15.6) died within 7 days, and 21.7% (95%CI 15.6-28.9) within 30 days. Regarding peptic ulcer, immediate hemostasis was achieved in 88% (95%CI 75-95), while 26% (95%CI 13-43) rebled. Higher ASA score was associated with mortality (OR 23.5, 95%CI 1.60-345; P=0.02). Immediate hemostasis was achieved in 100% of cases with malignancy and post-intervention bleeding, with rebleeding in 17% and 3.1%, respectively. Twenty-six patients received TC-325 for lower GI bleeding, and in all but one the primary outcome was achieved.
Conclusions: TC-325 monotherapy is safe and effective, especially in malignancy or post-endoscopic intervention bleeding. In patients with peptic ulcer, it could be helpful when the primary treatment is unfeasible, as bridge to definite therapy.
Keywords: Hemospray®; TC-325; endoscopy; upper gastrointestinal bleeding.
Copyright: © 2024 Hellenic Society of Gastroenterology.
Conflict of interest statement
Conflict of Interest: Rehan J. Haidry declares: Pentax Medical, Apollo Endosurgery, Medtronic, Odin Vision, Cook Endoscopy, Fractyl Limited, Endogastric Solutions; Enrique Rodríguez de Santiago declares: Olympus, Norgine and Apollo Endosurgery (Educational activities) Adacyte therapeuthics (Advisory); Seth A. Gross declares: Cook, Medtronic, Olympus, Microtech. The other authors have nothing to declare
Figures

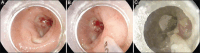
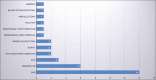
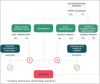
References
-
- Moudallel S, van den Eynde C, Malý J, Rydant S, Steurbaut S. Retrospective analysis of gastrointestinal bleedings with direct oral anticoagulants reported to EudraVigilance. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:1143–1153. - PubMed
-
- Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42-43:101610. - PubMed
-
- Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade:a nationwide analysis. Dig Dis Sci. 2018;63:1286–1293. - PubMed
-
- Button LA, Roberts SE, Evans PA, et al. Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007:a record linkage study. Aliment Pharmacol Ther. 2011;33:64–76. - PubMed
LinkOut - more resources
Full Text Sources
