Pharmacokinetics and Safety of Oliceridine Fumarate Injection in Chinese Patients with Chronic Non-Cancer Pain: A Phase I, Single-Ascending-Dose, Open-Label Clinical Trial
- PMID: 38974123
- PMCID: PMC11227858
- DOI: 10.2147/DDDT.S461416
Pharmacokinetics and Safety of Oliceridine Fumarate Injection in Chinese Patients with Chronic Non-Cancer Pain: A Phase I, Single-Ascending-Dose, Open-Label Clinical Trial
Abstract
Background: Oliceridine is a novel G protein-biased ligand μ-opioid receptor agonist. This study aimed to assess the pharmacokinetics and safety profile of single-ascending doses of oliceridine fumarate injection in Chinese patients with chronic non-cancer pain.
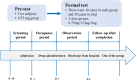
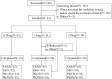
Methods: Conducted as a single-center, open-label trial, this study administered single doses of 0.75, 1.5, and 3.0 mg to 32 adult participants. The trial was conducted in two parts. First, we conducted a preliminary test comprising the administration of a single dose of 0.75mg to 2 participants. Then, we conducted the main trial involving intravenous administration of escalating doses of oliceridine fumarate (0.75 to 3 mg) to 30 participants. Pharmacokinetic (PK) parameters were derived using non-compartmental analysis. Additionally, the safety evaluation encompassed the monitoring of adverse events (AEs).
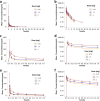
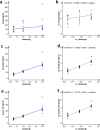
Results: 32 participants were included in the PK and safety analyses. Following a 2-min intravenous infusion of oliceridine fumarate injection (0.75, 1.5, or 3 mg), Cmax and Tmax ranged from 51.293 to 81.914 ng/mL and 0.034 to 0.083 h, respectively. AUC0-t and half-life (t1/2) increased more than proportionally with dosage (1.85-2.084 h). Treatment emergent adverse events (TEAEs) were found to be consistent with the commonly reported adverse effects of opioids, both post-administration and as documented in the original trials conducted in the United States. Critically, no serious adverse events were observed.
Conclusion: Oliceridine demonstrated comparable PK parameters and a consistent PK profile in the Chinese population, in line with the PK results observed in the original trials conducted in the United States. Oliceridine was safe and well tolerated in Chinese patients with chronic non-cancer pain at doses ranging from 0.75 mg to 3.0 mg.
Trial registration: The trial is registered at chictr.org.cn (ChiCTR2100047180).
Keywords: G-protein-biased ligand; chronic pain; oliceridine; pharmacokinetics; phase I trial.
© 2024 Ni et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures
References
-
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American society of anesthesiologists task force on acute pain management. Anesthesiology. 2012;116(2):248–273. doi:10.1097/ALN.0b013e31823c1030 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

