Crystal structure and Hirshfeld surface analysis of 2-bromo-ethyl-ammonium bromide - a possible side product upon synthesis of hybrid perovskites
- PMID: 38974162
- PMCID: PMC11223709
- DOI: 10.1107/S2056989024005619
Crystal structure and Hirshfeld surface analysis of 2-bromo-ethyl-ammonium bromide - a possible side product upon synthesis of hybrid perovskites
Abstract
This study presents the synthesis, characterization and Hirshfeld surface analysis of a small organic ammonium salt, C2H7BrN+·Br-. Small cations like the one in the title compound are considered promising components of hybrid perovskites, crucial for optoelectronic and photovoltaic applications. While the incorporation of this organic cation into various hybrid perovskite structures has been explored, its halide salt counterpart remains largely uninvestigated. The obtained structural results are valuable for the synthesis and phase analysis of hybrid perovskites. The title compound crystallizes in the solvent-free form in the centrosymmetric monoclinic space group P21/c, featuring one organic cation and one bromide anion in its asymmetric unit, with a torsion angle of -64.8 (2)° between the ammonium group and the bromine substituent, positioned in a gauche conformation. The crystal packing is predominantly governed by Br⋯H inter-actions, which constitute 62.6% of the overall close atom contacts.
Keywords: 2-bromoethylamine hydrobromide; Hirshfeld surface analysis; crystal structure; hybrid perovskite.
© Semenikhin et al. 2024.
Figures
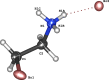
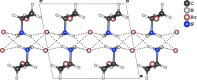


 , z +
, z +  ; (iii) −x + 1, y +
; (iii) −x + 1, y +  , −z +
, −z +  .]
.]
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Tailor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. 1–19.
-
- Dey, A., Ye, J., De, A., Debroye, E., Ha, S. K., Bladt, E., Kshirsagar, A. S., Wang, Z., Yin, J., Wang, Y., Quan, L. N., Yan, F., Gao, M., Li, X., Shamsi, J., Debnath, T., Cao, M., Scheel, M. A., Kumar, S., Steele, J. A., Gerhard, M., Chouhan, L., Xu, K., Wu, X. G., Li, Y., Zhang, Y., Dutta, A., Han, C., Vincon, I., Rogach, A. L., Nag, A., Samanta, A., Korgel, B. A., Shih, C. J., Gamelin, D. R., Son, D. H., Zeng, H., Zhong, H., Sun, H., Demir, H. V., Scheblykin, I. G., Mora-Seró, I., Stolarczyk, J. K., Zhang, J. Z., Feldmann, J., Hofkens, J., Luther, J. M., Pérez-Prieto, J., Li, L., Manna, L., Bodnarchuk, M. I., Kovalenko, M. V., Roeffaers, M. B. J., Pradhan, N., Mohammed, O. F., Bakr, O. M., Yang, P., Müller-Buschbaum, P., Kamat, P. V., Bao, Q., Zhang, Q., Krahne, R., Galian, R. E., Stranks, S. D., Bals, S., Biju, V., Tisdale, W. A., Yan, Y., Hoye, R. L. Z. & Polavarapu, L. (2021). ACS Nano, 15, 10775–10981.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
-
- Hassan, Y., Park, J. H., Crawford, M. L., Sadhanala, A., Lee, J., Sadighian, J. C., Mosconi, E., Shivanna, R., Radicchi, E., Jeong, M., Yang, C., Choi, H., Park, S. H., Song, M. H., De Angelis, F., Wong, C. Y., Friend, R. H., Lee, B. R. & Snaith, H. J. (2021). Nature, 591, 72–77. - PubMed
LinkOut - more resources
Full Text Sources
