Recyclable LaF3·Pd nanocatalyst in Suzuki coupling: green synthesis of biaryls from haloarenes and phenylboronic acids
- PMID: 38974224
- PMCID: PMC11224950
- DOI: 10.1039/d4ra00686k
Recyclable LaF3·Pd nanocatalyst in Suzuki coupling: green synthesis of biaryls from haloarenes and phenylboronic acids
Abstract
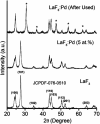
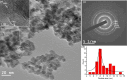
Herein we prepared the novel LaF3·Pd nanocatalyst characterized by XRD and TEM analysis. The nanocatalyst was applied in Suzuki coupling reaction for the synthesis of biaryls in aqueous medium from readily available aryl halides (bromides and iodides) and substituted phenylboronic acids in the presence of K2CO3 as the base at 70 °C. The present method is capable of giving the C-C coupled product in good to excellent yields (up to 97%). The reactions were conducted under green conditions in aqueous medium and the nanocatalyst used in this study was recyclable. The recyclability and reusability of the catalyst was checked for seven consecutive cycles without significant loss in reactivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Green and Sustainable Synthesis of Biaryls Using LaPO4·Pd Recyclable Nanocatalyst by the Suzuki-Miyaura Coupling in Aqueous Medium.ACS Omega. 2025 Jun 5;10(23):24105-24116. doi: 10.1021/acsomega.4c10467. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547644 Free PMC article.
-
A novel 4-aminoantipyrine-Pd(II) complex catalyzes Suzuki-Miyaura cross-coupling reactions of aryl halides.Beilstein J Org Chem. 2014 Dec 1;10:2821-6. doi: 10.3762/bjoc.10.299. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25550748 Free PMC article.
-
Pd-aminoclay nanocomposite as an efficient recyclable catalyst for hydrogenation and Suzuki cross coupling reactions.J Nanosci Nanotechnol. 2012 Mar;12(3):2000-7. doi: 10.1166/jnn.2012.6132. J Nanosci Nanotechnol. 2012. PMID: 22755012
-
Synthesis and characterization of Ni(II) complex functionalized silica-based magnetic nanocatalyst and its application in C-N and C-C cross-coupling reactions.Mol Divers. 2019 Aug;23(3):527-539. doi: 10.1007/s11030-018-9888-2. Epub 2018 Nov 22. Mol Divers. 2019. PMID: 30467803
-
Palladium nanoparticles embedded on mesoporous TiO2 material (Pd@MTiO2) as an efficient heterogeneous catalyst for Suzuki-Coupling reactions in water medium.J Colloid Interface Sci. 2017 Dec 15;508:378-386. doi: 10.1016/j.jcis.2017.08.046. Epub 2017 Aug 16. J Colloid Interface Sci. 2017. PMID: 28843927
Cited by
-
Green and Sustainable Synthesis of Biaryls Using LaPO4·Pd Recyclable Nanocatalyst by the Suzuki-Miyaura Coupling in Aqueous Medium.ACS Omega. 2025 Jun 5;10(23):24105-24116. doi: 10.1021/acsomega.4c10467. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547644 Free PMC article.
References
-
- Chandrasekhar S. Parida B. B. Rambabu C. J. Org. Chem. 2008;73:7826–7828. - PubMed
- Raiguru B. P. Panda J. Mohapatra S. Nayak S. J. Mol. Struct. 2023;1294:136282.
-
- Biffis A. Centomo P. Zottom A. D. Zecca M. Chem. Rev. 2018;118:2249–2295. - PubMed
-
- Niwa T. Uetake Y. Isoda M. Takimoto T. Nakaoka M. Hashizume D. Sakurai H. Hosoya T. Nat. Catal. 2021;4:1080–1088.
- Hussain I. Capricho J. Yawer M. A. Adv. Synth. Catal. 2016;358:3320–3349.
- Hooshmand S. E. Heidari B. Sedghi R. Varma R. S. Green Chem. 2019;21:381–405.
- Hassan A. Baghel A. S. Kumar A. Das N. Chem.–Asian J. 2023:e202300778. - PubMed
- Acharya S. S. Parida B. B. ChemistrySelect. 2024;9:e202305233.
LinkOut - more resources
Full Text Sources

