NAD+ metabolism and therapeutic strategies in cardiovascular diseases
- PMID: 38974325
- PMCID: PMC11223091
- DOI: 10.1016/j.athplu.2024.06.001
NAD+ metabolism and therapeutic strategies in cardiovascular diseases
Abstract
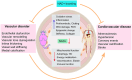
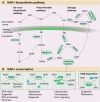
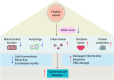
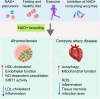
Nicotinamide adenine dinucleotide (NAD+) is a central and pleiotropic metabolite involved in cellular energy metabolism, cell signaling, DNA repair, and protein modifications. Cardiovascular diseases (CVDs) are the leading cause of death worldwide. Metabolic stress and aging directly affect the cardiovascular system. Compelling data suggest that NAD + levels decrease with age, obesity, and hypertension, which are all notable risk factors for CVD. In addition, the therapeutic elevation of NAD + levels reduces chronic low-grade inflammation, reactivates autophagy and mitochondrial biogenesis, and enhances oxidative metabolism in vascular cells of humans and rodents with vascular disorders. In preclinical models, NAD + boosting can also expand the health span, prevent metabolic syndrome, and decrease blood pressure. Moreover, NAD + storage by genetic, pharmacological, or natural dietary NAD + -increasing strategies has recently been shown to be effective in improving the pathophysiology of cardiac and vascular health in different animal models, and human health. Here, we review and discuss NAD + -related mechanisms pivotal for vascular health and summarize recent experimental evidence in NAD + research directly related to vascular disease, including atherosclerosis, and coronary artery disease. Finally, we comparatively assess distinct NAD + precursors for their clinical efficacy and the efficiency of NAD + elevation in the treatment of major CVD. These findings may provide ideas for new therapeutic strategies to prevent and treat CVD in the clinic.
Keywords: Atherosclerosis; Cardiovascular diseases; Nicotinamide adenine dinucleotide; Vascular disorder.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
[NAD+ metabolism in cardiovascular diseases].Sheng Li Xue Bao. 2025 Apr 25;77(2):345-360. doi: 10.13294/j.aps.2025.0034. Sheng Li Xue Bao. 2025. PMID: 40326077 Review. Chinese.
-
NAD+ and Vascular Dysfunction: From Mechanisms to Therapeutic Opportunities.J Lipid Atheroscler. 2022 May;11(2):111-132. doi: 10.12997/jla.2022.11.2.111. Epub 2022 Apr 6. J Lipid Atheroscler. 2022. PMID: 35656147 Free PMC article. Review.
-
Therapeutic Potential of Emerging NAD+-Increasing Strategies for Cardiovascular Diseases.Antioxidants (Basel). 2021 Dec 3;10(12):1939. doi: 10.3390/antiox10121939. Antioxidants (Basel). 2021. PMID: 34943043 Free PMC article. Review.
-
NAD+ Metabolism in Cardiac Health, Aging, and Disease.Circulation. 2021 Nov 30;144(22):1795-1817. doi: 10.1161/CIRCULATIONAHA.121.056589. Epub 2021 Nov 29. Circulation. 2021. PMID: 34843394
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
Cited by
-
Nicotinamide Riboside-Driven Modulation of SIRT3/mtROS/JNK Signaling Pathways Alleviates Myocardial Ischemia-Reperfusion Injury.Int J Med Sci. 2024 Aug 12;21(11):2139-2148. doi: 10.7150/ijms.97530. eCollection 2024. Int J Med Sci. 2024. PMID: 39239543 Free PMC article.
References
-
- Chung M.K., Eckhardt L.L., Chen L.Y., Ahmed H.M., Gopinathannair R., Joglar J.A., Noseworthy P.A., Pack Q.R., Sanders P., Trulock K.M. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American heart association. Circulation. 2020;141:e750–e772. doi: 10.1161/CIR.0000000000000748. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
