Polypill versus medication monotherapy in the prevention of cardiovascular diseases in Iran: An economic evaluation study
- PMID: 38974330
- PMCID: PMC11225077
- DOI: 10.1002/hsr2.2240
Polypill versus medication monotherapy in the prevention of cardiovascular diseases in Iran: An economic evaluation study
Abstract
Background and aims: Cardiovascular diseases (CVDs) are one of the major diseases in developing and developed countries and have high prevalence and mortality rates. Pharmacological interventions, especially the use of combination medications, can have preventive effects in patients with CVDs. Recently, in the PolyIran trial, a combination of atorvastatin, hydrochlorothiazide, aspirin, and valsartan or enalapril (Polypill) was shown to be effective in providing survival benefits as a primary prevention strategy. In the present study, we examine the cost-effectiveness of the use of polypill compared to its individual components (named as medication monotherapy) in the prevention of CVDs in Iran.
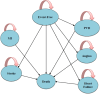
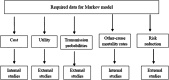
Methods: This was an economic evaluation study conducted to compare the cost-utility of polypill with that of medication monotherapy for 10,000 hypothetical cohorts of people over 35 years of age using the Markov model and with a lifetime horizon. The study perspective was patient perspective and direct medical costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio were estimated. To deal with uncertaintysensitivity analyses were used.
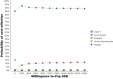
Results: The results showed that polypill, with the lowest costs (871 USD) and highest QALYs (14.55), had the most cost-utility than medication monotherapy. Also, the results showed that the highest sensitivities were related to the utilities of angina and stroke states. At the 21,768 USD threshold, polypill had a 92% probability of being cost-effective versus other medications.
Conclusion: Considering that polypill had the most cost-utility, it is suggested that health system policymakers pay special attention to polypill in designing clinical guidelines. Also, through covering this medication by health insurance organizations, it is possible to complete the country's medicine pharmacopeia in preventing CVDs.
Keywords: Iran; Markov model; Polypill; cardiovascular diseases; cost‐utility analysis.
© 2024 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial.Lancet. 2019 Aug 24;394(10199):672-683. doi: 10.1016/S0140-6736(19)31791-X. Lancet. 2019. PMID: 31448738 Clinical Trial.
-
Polypill for the prevention of cardiovascular disease (PolyIran): study design and rationale for a pragmatic cluster randomized controlled trial.Eur J Prev Cardiol. 2015 Dec;22(12):1609-17. doi: 10.1177/2047487314550803. Epub 2014 Sep 17. Eur J Prev Cardiol. 2015. PMID: 25230980 Free PMC article. Clinical Trial.
-
Usefulness of a Cardiovascular Polypill in the Treatment of Secondary Prevention Patients in Spain: A Cost-effectiveness Study.Rev Esp Cardiol (Engl Ed). 2017 Jan;70(1):42-49. doi: 10.1016/j.rec.2016.05.009. Epub 2016 Jul 26. Rev Esp Cardiol (Engl Ed). 2017. PMID: 27474481 English, Spanish.
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
-
Cost-effectiveness of fixed-dose combination pill (Polypill) in primary and secondary prevention of cardiovascular disease: A systematic literature review.PLoS One. 2022 Jul 28;17(7):e0271908. doi: 10.1371/journal.pone.0271908. eCollection 2022. PLoS One. 2022. PMID: 35901100 Free PMC article.
References
-
- World Health Organization . Coronavirus disease 2019 (COVID‐19): situation report. Vol 114. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/332089
LinkOut - more resources
Full Text Sources

