Cbl and Cbl-b ubiquitin ligases are essential for intestinal epithelial stem cell maintenance
- PMID: 38974465
- PMCID: PMC11225835
- DOI: 10.1016/j.isci.2024.109912
Cbl and Cbl-b ubiquitin ligases are essential for intestinal epithelial stem cell maintenance
Abstract
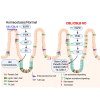
Receptor tyrosine kinases (RTKs) control stem cell maintenance vs. differentiation decisions. Casitas B-lineage lymphoma (CBL) family ubiquitin ligases are negative regulators of RTKs, but their stem cell regulatory roles remain unclear. Here, we show that Lgr5+ intestinal stem cell (ISC)-specific inducible Cbl-knockout (KO) on a Cblb null mouse background (iDKO) induced rapid loss of the Lgr5 Hi ISCs with transient expansion of the Lgr5 Lo transit-amplifying population. LacZ-based lineage tracing revealed increased ISC commitment toward enterocyte and goblet cell fate at the expense of Paneth cells. Functionally, Cbl/Cblb iDKO impaired the recovery from radiation-induced intestinal epithelial injury. In vitro, Cbl/Cblb iDKO led to inability to maintain intestinal organoids. Single-cell RNA sequencing in organoids identified Akt-mTOR (mammalian target of rapamycin) pathway hyperactivation upon iDKO, and pharmacological Akt-mTOR axis inhibition rescued the iDKO defects. Our results demonstrate a requirement for Cbl/Cblb in the maintenance of ISCs by fine-tuning the Akt-mTOR axis to balance stem cell maintenance vs. commitment to differentiation.
Keywords: Biochemistry; Biological sciences; Cell biology; Molecular biology; Natural sciences; Stem cells research.
© 2024 The Author(s).
Conflict of interest statement
H.B. and V.B. received funding from Nimbus Therapeutics for an unrelated project.
Figures



Update of
-
Cbl and Cbl-b Ubiquitin Ligases are Essential for Intestinal Epithelial Stem Cell Maintenance.bioRxiv [Preprint]. 2023 May 22:2023.05.17.541154. doi: 10.1101/2023.05.17.541154. bioRxiv. 2023. Update in: iScience. 2024 May 08;27(6):109912. doi: 10.1016/j.isci.2024.109912. PMID: 37292716 Free PMC article. Updated. Preprint.
References
-
- Ramachandra C.J.A., Mehta A., Lua C.H., Chitre A., Ja K.P.M.M., Shim W. ErbB Receptor Tyrosine Kinase: A Molecular Switch Between Cardiac and Neuroectoderm Specification in Human Pluripotent Stem Cells. Stem Cell. 2016;34:2461–2470. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

