Single-cell view into the role of microbiota shaping host immunity in the larynx
- PMID: 38974468
- PMCID: PMC11225822
- DOI: 10.1016/j.isci.2024.110156
Single-cell view into the role of microbiota shaping host immunity in the larynx
Abstract
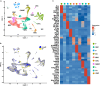
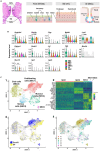
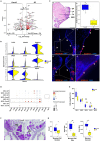
Microbiota play a critical role in the development and training of host innate and adaptive immunity. We present the cellular landscape of the upper airway, specifically the larynx, by establishing a reference single-cell atlas, while dissecting the role of microbiota in cell development and function at single-cell resolution. We highlight the larynx's cellular heterogeneity with the identification of 16 cell types and 34 distinct subclusters. Our data demonstrate that commensal microbiota have extensive impact on the laryngeal immune system by regulating cell differentiation, increasing the expression of genes associated with host defense, and altering gene regulatory networks. We uncover macrophages, innate lymphoid cells, and multiple secretory epithelial cells, whose cell proportions and expressions vary with microbial exposure. These cell types play pivotal roles in maintaining laryngeal and upper airway health and provide specific guidance into understanding the mechanism of immune system regulation by microbiota in laryngeal health and disease.
Keywords: Immunity; Microbiome; Transcriptomics.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
T-bet expressing Tr1 cells driven by dietary signals dominate the small intestinal immune landscape.bioRxiv [Preprint]. 2025 Jul 4:2025.06.30.662190. doi: 10.1101/2025.06.30.662190. bioRxiv. 2025. PMID: 40747421 Free PMC article. Preprint.
-
Elimination of gut microbiota hinders the therapeutic effect of amentoflavone on respiratory syncytial virus-induced lung inflammation injury by regulating innate immunity.Phytomedicine. 2025 Sep;145:157033. doi: 10.1016/j.phymed.2025.157033. Epub 2025 Jul 8. Phytomedicine. 2025. PMID: 40701130
-
Impact of the maternal microbiome on neonatal immune development.J Reprod Immunol. 2025 Aug;170:104542. doi: 10.1016/j.jri.2025.104542. Epub 2025 May 16. J Reprod Immunol. 2025. PMID: 40403512 Review.
-
Microbiota-dependent modulation of intestinal anti-inflammatory CD4+ T cell responses.Semin Immunopathol. 2025 Apr 1;47(1):23. doi: 10.1007/s00281-025-01049-6. Semin Immunopathol. 2025. PMID: 40167791 Review.
-
Comprehensive Single-Cell RNA Atlas of Human Laryngeal Normal, Preneoplastic, and Tumorigenic States.Clin Cancer Res. 2025 Aug 1;31(15):3332-3343. doi: 10.1158/1078-0432.CCR-24-3679. Clin Cancer Res. 2025. PMID: 40407728 Free PMC article.
Cited by
-
Age and sex-related variations in murine laryngeal microbiota.PLoS One. 2024 May 14;19(5):e0300672. doi: 10.1371/journal.pone.0300672. eCollection 2024. PLoS One. 2024. PMID: 38743725 Free PMC article.
-
Effects of psychosocial stress on laryngeal microbiology and epithelial barrier integrity.Sci Rep. 2025 Jul 12;15(1):25278. doi: 10.1038/s41598-025-10170-3. Sci Rep. 2025. PMID: 40652088 Free PMC article.
-
Biophysical aspects of mechanotransduction in cells and their physiological/biological implications in vocal fold vibration: a narrative review.Front Cell Dev Biol. 2025 Jan 27;13:1501341. doi: 10.3389/fcell.2025.1501341. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 39931244 Free PMC article. Review.
-
Cellular heterogeneity and patterning strategies as revealed by upper respiratory epithelium single cell atlas.iScience. 2025 Jun 7;28(7):112845. doi: 10.1016/j.isci.2025.112845. eCollection 2025 Jul 18. iScience. 2025. PMID: 40612507 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

