Lysosomal TRPML1 triggers global Ca2+ signals and nitric oxide release in human cerebrovascular endothelial cells
- PMID: 38974517
- PMCID: PMC11224436
- DOI: 10.3389/fphys.2024.1426783
Lysosomal TRPML1 triggers global Ca2+ signals and nitric oxide release in human cerebrovascular endothelial cells
Abstract
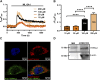
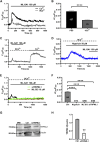
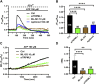
Lysosomal Ca2+ signaling is emerging as a crucial regulator of endothelial Ca2+ dynamics. Ca2+ release from the acidic vesicles in response to extracellular stimulation is usually promoted via Two Pore Channels (TPCs) and is amplified by endoplasmic reticulum (ER)-embedded inositol-1,3,4-trisphosphate (InsP3) receptors and ryanodine receptors. Emerging evidence suggests that sub-cellular Ca2+ signals in vascular endothelial cells can also be generated by the Transient Receptor Potential Mucolipin 1 channel (TRPML1) channel, which controls vesicle trafficking, autophagy and gene expression. Herein, we adopted a multidisciplinary approach, including live cell imaging, pharmacological manipulation, and gene targeting, revealing that TRPML1 protein is expressed and triggers global Ca2+ signals in the human brain microvascular endothelial cell line, hCMEC/D3. The direct stimulation of TRPML1 with both the synthetic agonist, ML-SA1, and the endogenous ligand phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) induced a significant increase in [Ca2+]i, that was reduced by pharmacological blockade and genetic silencing of TRPML1. In addition, TRPML1-mediated lysosomal Ca2+ release was sustained both by lysosomal Ca2+ release and ER Ca2+- release through inositol-1,4,5-trisphophate receptors and store-operated Ca2+ entry. Notably, interfering with TRPML1-mediated lysosomal Ca2+ mobilization led to a decrease in the free ER Ca2+ concentration. Imaging of DAF-FM fluorescence revealed that TRPML1 stimulation could also induce a significant Ca2+-dependent increase in nitric oxide concentration. Finally, the pharmacological and genetic blockade of TRPML1 impaired ATP-induced intracellular Ca2+ release and NO production. These findings, therefore, shed novel light on the mechanisms whereby the lysosomal Ca2+ store can shape endothelial Ca2+ signaling and Ca2+-dependent functions in vascular endothelial cells.
Keywords: Ca2+ signaling; InsP3 receptors; TRPML1; endothelial cells; lysosomes; nitric oxide; store-operated Ca2+ entry.
Copyright © 2024 Brunetti, Berra-Romani, Conca, Soda, Biella, Gerbino, Moccia and Scarpellino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Figures




References
-
- Bader A., Bintig W., Begandt D., Klett A., Siller I. G., Gregor C., et al. (2017). Adenosine receptors regulate gap junction coupling of the human cerebral microvascular endothelial cells hCMEC/D3 by Ca(2+) influx through cyclic nucleotide-gated channels. J. Physiol. 595 (8), 2497–2517. 10.1113/JP273150 - DOI - PMC - PubMed
-
- Berra-Romani R., Brunetti V., Pellavio G., Soda T., Laforenza U., Scarpellino G., et al. (2023). Allyl isothiocianate induces Ca(2+) signals and nitric oxide release by inducing reactive oxygen species production in the human cerebrovascular endothelial cell line hCMEC/D3. Cells 12 (13), 1732. 10.3390/cells12131732 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

