Screening and surveillance of hepatocellular carcinoma by serum des-gamma-carboxy prothrombin in patients with glycogen storage disease type Ia
- PMID: 38974608
- PMCID: PMC11224497
- DOI: 10.1002/jmd2.12414
Screening and surveillance of hepatocellular carcinoma by serum des-gamma-carboxy prothrombin in patients with glycogen storage disease type Ia
Abstract
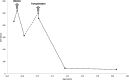
No sensitive tumor marker for hepatocellular carcinoma (HCC) is available for patients with glycogen storage disease type Ia (GSDIa), in whom alpha-fetoprotein and carcino-embryonic antigen levels often remain normal. We describe increased levels of the HCC tumor marker des-gamma-carboxy prothrombin (DCP) in GSDIa patients with HCC. In one case DCP levels normalized after liver transplantation. We recommend including DCP as a screening HCC tumor marker in the surveillance of patients with GSDIa.
Keywords: des‐gamma‐carboxy prothrombin; glycogen storage disease type I; hepatocellular adenoma; hepatocellular carcinoma; tumor markers.
© 2024 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Andrea Schreuder declares that she experiences no competing interest concerning the content of this manuscript. However, in the past 36 months, there have been confidentiality agreements with third parties. For all private–public relationships, all contracts are via UMCG Contract Research Desk, and all payments are to UMCG. Ruben Overduin declares that he experiences no competing interests concerning the content of this manuscript. There is a consultation agreement with Ultragenyx Pharmaceutical Inc of which the contract is via UMCG Contract Research Desk and all payments are to UMCG. Chantal Peltenburg declares that she experiences no competing interest concerning the content of this manuscript. However, she is an independent investigator for study sponsored by Clinuvel. Lonneke de Boer declares that she experiences no competing interest concerning the content of this manuscript. Frank Bodewes declares that he experiences no competing interests concerning the content of this manuscript. Terry Derks declares that he experiences no competing interests concerning the content of this manuscript. However, there are confidentiality agreements with third parties. In the past 36 months, there have been consultation agreements (with Danone, Ultragenyx Pharmaceutical Inc, ModernaTX Inc, and Beam Therapeutics), contracts for financial research support for investigator‐initiated research (NCT04311307) and sponsor‐initiated research (NCT03517085, NCT03970278, NCT05139316, and NCT05196165), honoraria for lectures or presentations (by MEDTalks, Prelum, and Danone), and participations in a Data Safety Monitoring Board (NCT05095727) and Advisory Boards (Ultragenyx Pharmaceutical Inc, ModernaTX Inc, and Beam Therapeutics). For all private‐public relationships, all contracts are via UMCG Contract Research Desk, and all payments are to UMCG.
Figures
References
-
- Bali DS, El‐Gharbawy A, Austin S, Pendyal S, Kishnani PS. Glycogen storage disease type I. GeneReviews®. Published online. 2021;1‐28. https://www.ncbi.nlm.nih.gov/books/NBK1312/
-
- Lamerz R, Runge M, Stieber P, Meissner E. Use of serum PIVKA‐II (DCP) determination for differentiation between benign and malignant liver diseases. Anticancer Res. 1999;(19):2489‐2493. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous