Attenuated mutants of Salmonella enterica Typhimurium mediate melanoma regression via an immune response
- PMID: 38974834
- PMCID: PMC11224151
- DOI: 10.3389/ebm.2024.10081
Attenuated mutants of Salmonella enterica Typhimurium mediate melanoma regression via an immune response
Abstract
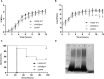
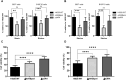
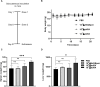
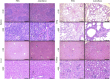
The lack of effective treatment options for an increasing number of cancer cases highlights the need for new anticancer therapeutic strategies. Immunotherapy mediated by Salmonella enterica Typhimurium is a promising anticancer treatment. Candidate strains for anticancer therapy must be attenuated while retaining their antitumor activity. Here, we investigated the attenuation and antitumor efficacy of two S. enterica Typhimurium mutants, ΔtolRA and ΔihfABpmi, in a murine melanoma model. Results showed high attenuation of ΔtolRA in the Galleria mellonella model, and invasion and survival in tumor cells. However, it showed weak antitumor effects in vitro and in vivo. Contrastingly, lower attenuation of the attenuated ΔihfABpmi strain resulted in regression of tumor mass in all mice, approximately 6 days after the first treatment. The therapeutic response induced by ΔihfABpmi was accompanied with macrophage accumulation of antitumor phenotype (M1) and significant increase in the mRNAs of proinflammatory mediators (TNF-α, IL-6, and iNOS) and an apoptosis inducer (Bax). Our findings indicate that the attenuated ΔihfABpmi exerts its antitumor activity by inducing macrophage infiltration or reprogramming the immunosuppressed tumor microenvironment to an activated state, suggesting that attenuated S. enterica Typhimurium strains based on nucleoid-associated protein genes deletion could be immunotherapeutic against cancer.
Keywords: S. enterica Typhimurium; anticancer; macrophage; melanoma; mutants.
Copyright © 2024 Pérez Jorge, Gontijo, Silva, Goes, Jaimes-Florez, Coser, Rocha, Giorgio and Brocchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


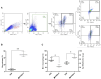
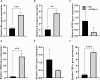
References
-
- Eggermont AMM, Bellomo D, Arias-Mejias SM, Quattrocchi E, Sominidi-Damodaran S, Bridges AG, et al. Identification of stage I/IIA melanoma patients at high risk for disease relapse using a clinicopathologic and gene expression model. Eur J Cancer (2020) 140:11–8. 10.1016/j.ejca.2020.08.029 - DOI - PMC - PubMed
-
- Boardman CH, Brady WE, Dizon DS, Kunos CA, Moore KN, Zanotti KM, et al. A phase I evaluation of extended field radiation therapy with concomitant cisplatin chemotherapy followed by paclitaxel and carboplatin chemotherapy in women with cervical carcinoma metastatic to the para-aortic lymph nodes: an NRG oncology/gynecologic oncology group study. Gynecol Oncol (2018) 151:202–7. 10.1016/j.ygyno.2018.08.006 - DOI - PMC - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

