Omega-3 blood biomarkers relate to brain glucose uptake in individuals at risk of Alzheimer's disease dementia
- PMID: 38974876
- PMCID: PMC11224768
- DOI: 10.1002/dad2.12596
Omega-3 blood biomarkers relate to brain glucose uptake in individuals at risk of Alzheimer's disease dementia
Abstract
Introduction: Brain glucose hypometabolism is a preclinical feature of Alzheimer's disease (AD). Dietary omega-3 fatty acids promote brain glucose metabolism, but clinical research is incipient. Circulating omega-3s objectively reflect their dietary intake.
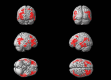
Methods: This was a cross-sectional study in 320 cognitively unimpaired participants at increased risk of AD dementia. Using lipidomics, we determined blood docosahexaenoic (DHA) and alpha-linolenic (ALA) acid levels (omega-3s from marine and plant origin, respectively). We assessed brain glucose metabolism using [18-F]-fluorodeoxyglucose (FDG) positron emission tomography (PET).
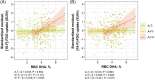
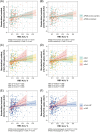
Results: Blood ALA directly related to FDG uptake in brain areas known to be affected in AD. Stronger associations were observed in apolipoprotein E ε4 carriers and homozygotes. For DHA, significant direct associations were restricted to amyloid beta-positive tau-positive participants.
Discussion: Blood omega-3 directly relate to preserved glucose metabolism in AD-vulnerable brain regions in individuals at increased risk of AD dementia. This adds to the benefits of omega-3 supplementation in the preclinical stage of AD dementia.
Highlights: Blood omega-3s were related to brain glucose uptake in participants at risk of Alzheimer's disease (AD) dementia.Complementary associations were observed for omega-3 from marine and plant sources.Foods rich in omega-3 might be useful in early features of AD.
Keywords: biomarkers; diet; fatty fish; fish oil; nuts; n‐3 fatty acids; polyunsaturated fatty acids; walnuts.
© 2024 The Author(s). Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
MSC has given lectures in symposia sponsored by Roche Diagnostics, S.L.U. Roche Farma, S.A., and Amirall;and he has served as a consultant and at advisory boards for Roche Diagnostics International Ltd and Grifols S.L. He was granted with a project funded by Roche Diagnostics International Ltd; payments were made to the institution (BBRC). He received in‐kind support for research (to the institution) from Roche Diagnostics International Ltd, Avid Radiopharmaceuticals, Inc., Eli Lilly, and Janssen Research & Development. JLM is currently a full‑time employee of H. Lundbeck A/S and previously served as a consultant or on advisory boards for the following for‑profit companies or has given lectures in symposia sponsored by the following for‑profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, and ProMIS Neurosciences. JDG receives research funding from Roche Diagnostics and GE Healthcare and has given lectures at symposia sponsored by Biogen and Philips. AS‐V has received research grant funding through his institution and support to attend professional meetings from the California Walnut Commission. Other authors report no conflicts of interest. Disclosures are available in the supporting information.
Figures
References
LinkOut - more resources
Full Text Sources
