Nomogram for predicting pathologic complete response following preoperative chemoradiotherapy in patients with esophageal squamous cell carcinoma
- PMID: 38974878
- PMCID: PMC11227365
- DOI: 10.1093/gastro/goae060
Nomogram for predicting pathologic complete response following preoperative chemoradiotherapy in patients with esophageal squamous cell carcinoma
Abstract
Background: In patients with esophageal squamous cell carcinoma (ESCC), accurately predicting a pathologic complete response (pCR) to preoperative chemoradiotherapy (PCRT) has the potential to enable an active surveillance strategy without esophagectomy. We aimed to establish a reliable multiparameter nomogram model that combines tumor characteristics, imaging modalities, and hematologic markers to predict pCR in patients with ESCC who underwent PCRT and esophagectomy.
Methods: We retrospectively reviewed the medical records of 457 patients with ESCC who received PCRT followed by esophagectomy between January 2005 and October 2020. The nomogram model was developed using logistic regression analysis with a training cohort and externally validated with a validation cohort.
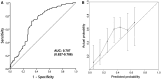
Results: In the training and validation cohorts, 44.2% (126/285) and 48.3% (83/172) of patients, respectively, achieved pCR after PCRT. The 5-year rates of overall survival, progression-free survival, and freedom from local progression in the training cohort were 51.6%, 48.5%, and 77.6%, respectively. The parameters included in the nomogram were histologic grade, clinical N stage, maximum standardized uptake value on positron emission tomography, and post-PCRT biopsy. Hematologic markers were significantly associated with survival outcomes but not with pCR. The area under the receiver operating characteristic curve of the nomogram was 0.717, 0.704, and 0.707 for the training cohort, internal validation cohort, and external validation cohort, respectively.
Conclusion: Our nomogram model based on four parameters obtained from standard clinical practice demonstrated good performance in both the training and validation cohorts and could be useful to aid clinical decision-making to determine whether surgery or active surveillance strategy should be pursued.
Keywords: chemoradiotherapy; esophageal squamous cell carcinoma; esophagectomy; nomograms; treatment outcome.
© The Author(s) 2024. Published by Oxford University Press and Sixth Affiliated Hospital of Sun Yat-sen University.
Conflict of interest statement
We have no conflicts of interest to declare.
Figures




References
-
- Shapiro J, van Lanschot JJB, Hulshof M. et al. ; CROSS Study Group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. - PubMed
-
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
LinkOut - more resources
Full Text Sources

