MBNL2 promotes aging-related cardiac fibrosis via inhibited SUMOylation of Krüppel-like factor4
- PMID: 38974966
- PMCID: PMC11226984
- DOI: 10.1016/j.isci.2024.110163
MBNL2 promotes aging-related cardiac fibrosis via inhibited SUMOylation of Krüppel-like factor4
Abstract
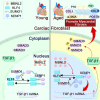
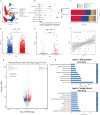
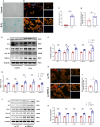
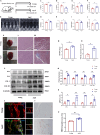
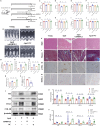
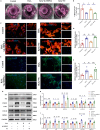
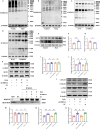
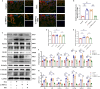
Aging-related cardiac fibrosis represents the principal pathological progression in cardiovascular aging. The Muscleblind-like splicing regulator 2 (MBNL2) has been unequivocally established as being associated with cardiovascular diseases. Nevertheless, its role in aging-related cardiac fibrosis remains unexplored. This investigation revealed an elevation of MBNL2 levels in the aged heart and senescent cardiac fibroblasts. Notably, the inhibition of MBNL2 demonstrated a capacity to mitigate H2O2-induced myofibroblast transformation and aging-related cardiac fibrosis. Further mechanistic exploration unveiled that aging heightened the expression of SENP1 and impeded the SUMO1 binding with KLF4, and SUMOylation of KLF4 effectively increased by the inhibition of MBNL2. Additionally, the inhibition of TGF-β1/SMAD3 signaling attenuated the impact of over-expression of MBNL2 in inducing senescence and cardiac fibrosis. MBNL2, by orchestrating SUMOylation of KLF4, upregulating the TGF-β1/SMAD3 signaling pathway, emerges as a significant promoter of aging-related cardiac fibrosis. This discovery identifies a novel regulatory target for managing aging-related cardiac fibrosis.
Keywords: Transcriptomics; molecular biology; omics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Glucosamine impedes transforming growth factor β1-mediated corneal fibroblast differentiation by targeting Krüppel-like factor 4.J Biomed Sci. 2019 Oct 9;26(1):72. doi: 10.1186/s12929-019-0566-1. J Biomed Sci. 2019. PMID: 31597574 Free PMC article.
-
FSTL1 promotes alveolar epithelial cell aging and worsens pulmonary fibrosis by affecting SENP1-mediated DeSUMOylation.Cell Biol Int. 2023 Oct;47(10):1716-1727. doi: 10.1002/cbin.12062. Epub 2023 Jun 27. Cell Biol Int. 2023. PMID: 37369969
-
Krüppel-like factor 4 transcriptionally regulates TGF-β1 and contributes to cardiac myofibroblast differentiation.PLoS One. 2013 Apr 30;8(4):e63424. doi: 10.1371/journal.pone.0063424. Print 2013. PLoS One. 2013. PMID: 23646205 Free PMC article.
-
Senescent cardiac fibroblasts: A key role in cardiac fibrosis.Biochim Biophys Acta Mol Basis Dis. 2023 Apr;1869(4):166642. doi: 10.1016/j.bbadis.2023.166642. Epub 2023 Jan 18. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 36669578 Review.
-
The Senescent Heart-"Age Doth Wither Its Infinite Variety".Int J Mol Sci. 2024 Mar 22;25(7):3581. doi: 10.3390/ijms25073581. Int J Mol Sci. 2024. PMID: 38612393 Free PMC article. Review.
Cited by
-
Functions of the Muscleblind-like protein family and their role in disease.Cell Commun Signal. 2025 Feb 18;23(1):97. doi: 10.1186/s12964-025-02102-5. Cell Commun Signal. 2025. PMID: 39966885 Free PMC article. Review.
-
CheekAge, a next-generation epigenetic buccal clock, is predictive of mortality in human blood.Front Aging. 2024 Oct 1;5:1460360. doi: 10.3389/fragi.2024.1460360. eCollection 2024. Front Aging. 2024. PMID: 39411517 Free PMC article.
-
Exploring the Impact of Microgravity on Gene Expression: Dysregulated Pathways and Candidate Repurposed Drugs.Int J Mol Sci. 2025 Feb 2;26(3):1287. doi: 10.3390/ijms26031287. Int J Mol Sci. 2025. PMID: 39941055 Free PMC article.
-
DsbA-L activates TGF-β1/SMAD3 signaling and M2 macrophage polarization by stimulating AKT1 and NLRP3 to promote pulmonary fibrosis.Mol Med. 2024 Nov 23;30(1):228. doi: 10.1186/s10020-024-00983-9. Mol Med. 2024. PMID: 39580448 Free PMC article.
References
LinkOut - more resources
Full Text Sources

