Safety and efficacy of selumetinib in pediatric and adult patients with neurofibromatosis type 1 and plexiform neurofibroma
- PMID: 38975694
- PMCID: PMC11630552
- DOI: 10.1093/neuonc/noae121
Safety and efficacy of selumetinib in pediatric and adult patients with neurofibromatosis type 1 and plexiform neurofibroma
Abstract
Background: The MEK inhibitor, selumetinib, reduces plexiform neurofibroma (PN) in pediatric patients with neurofibromatosis type 1 (NF1). Its safety and efficacy in adults with PN and effectiveness in other NF1 manifestations (eg, neurocognitive function, growth reduction, and café-au-lait spots) are unknown.
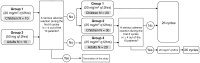
Methods: This open-label, phase II trial enrolled 90 pediatric or adult NF1 patients with inoperable, symptomatic, or potentially morbid, measurable PN (≥3 cm). Selumetinib was administered at doses of 20 or 25 mg/m2 or 50 mg q 12 hours for 2 years. Pharmacokinetics, PN volume, growth parameters, neurocognitive function, café-au-lait spots, and quality of life (QoL) were evaluated.
Results: Fifty-nine children and 30 adults (median age, 16 years; range, 3-47) received an average of 22 ± 5 (4-26) cycles of selumetinib. Eighty-eight (98.9%) out of 89 per-protocol patients showed volume reduction in the target PN (median, 40.8%; 4.2%-92.2%), and 81 (91%) patients showed partial response (≥20% volume reduction). The response lasted until cycle 26. Scores of neurocognitive functions (verbal comprehension, perceptual reasoning, processing speed, and full-scale IQ) significantly improved in both pediatric and adult patients (P < .05). Prepubertal patients showed increases in height score and growth velocity (P < .05). Café-au-lait spot intensity decreased significantly (P < .05). Improvements in QoL and pain scores were observed in both children and adults. All adverse events were CTCAE grade 1 or 2 and were successfully managed without drug discontinuation.
Conclusions: Selumetinib decreases PN volume in the majority of pediatric and adult NF1 patients while also showing efficacy in nonmalignant diverse NF1 manifestations.
Trial registration: Cris.nih.go.kr Identifier (KCT0003700).
Keywords: Café-au-Lait spots; mitogen-activated protein kinase kinases; neurofibroma; neurofibromatosis 1; plexiform.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
HK, and BHL have participated on an Advisory Board on a Round Table Discussion of Selumetinib for Astrazeneca.
Figures



References
-
- Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. - PubMed
-
- Xu GF, O’Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62(3):599–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

