Treat-and-Extend Versus Pro re nata Regimens of Ranibizumab and Aflibercept in Neovascular Age-Related Macular Degeneration: A Comparative Study from Routine Clinical Practice
- PMID: 38976148
- PMCID: PMC11341503
- DOI: 10.1007/s40123-024-00983-2
Treat-and-Extend Versus Pro re nata Regimens of Ranibizumab and Aflibercept in Neovascular Age-Related Macular Degeneration: A Comparative Study from Routine Clinical Practice
Abstract
Introduction: Anti-vascular endothelial growth factor (VEGF) is generally given using pro re nata or "treat-and-extend" (T&E) regimens for neovascular age-related macular degeneration (nAMD). Randomized clinical trials have reported that T&E is superior to Pro re nata (PRN), but results from clinical trials may not always be replicated in clinical practice. Real-world data comparing T&E and PRN regimens for nAMD are limited. The objective of this work was to report 24-month outcomes of PRN versus T&E regimens for ranibizumab and aflibercept to treat nAMD in routine clinical practice.
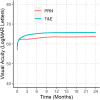
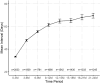
Methods: We conducted a retrospective analysis of data from a prospectively designed observational outcomes registry, the Fight Retinal Blindness! Project (FRB). Treatment-naïve eyes starting nAMD treatment with at least three injections using a T&E or PRN regimen were tracked by using the FRB. The primary outcome was the mean change in visual acuity (VA) measured by the number of letters read on a logarithm of the minimum angle of resolution chart at 2 years versus baseline. The secondary outcome was the number of injections at 2 years.
Results: From January 1, 2015 to January 31, 2019, 3313 eyes from 2948 patients with nAMD were included: 1243 eyes from 1065 patients were classified as PRN and 2070 eyes from 1935 patients started a T&E regimen. At 24 months, patients on the T&E regimen experienced significantly greater mean (95% confidence interval) improvement in VA than those on PRN (+ 4.2 [3.1, 5.2] vs. + 1.3 [0.1, 2.6] letters; p < 0.001), with more injections (14.9 standard deviation(SD) 4.3) vs. 9.8(SD 4.3); p < 0.001).
Conclusions: Eyes treated with a T&E regimen had better VA outcomes from VEGF inhibitors than eyes treated PRN. This large real-world data assessment supports previous data from randomized clinical trials that the T&E regimen delivers better outcomes than PRN.
Keywords: Intraocular injection; Neovascular AMD; Pro re nata; Treat-and-extend.
Plain language summary
This study focused on comparing two methods of treating neovascular age-related macular degeneration, a common eye condition. The treatments used were ranibizumab and aflibercept. We looked at the reactive “pro re nata” method, where treatment is given sporadically and only when the condition reactivates, and the proactive “treat-and-extend” method, which aims to keep the disease inactive with the fewest treatments at regular intervals. The main aim was to determine which method provides the best vision outcomes over a 24-month period and the frequency of treatment required. We found that the treat-and-extend method resulted in a greater improvement in vision than the pro re nata method, although it did require more injections. This study highlights the effectiveness of the treat-and-extend method for neovascular age-related macular degeneration, suggesting it gets better outcomes despite requiring more injections.
© 2024. The Author(s).
Conflict of interest statement
Eloi Debourdeau, Hélène Beylerian, Vincent Daien, Vuong Nguyen, Mark Gillies, Daniel Barthelmes, Catherine Creuzot-Garcher, Pierre Henry Gabrielle, Stela Vujosevic, Louise O’Toole, Martin Puzo and Benjamin Wolff have nothing to disclose.
Figures

References
-
- IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–411. 10.1016/j.ophtha.2012.04.015 - DOI - PubMed

