A Canadian Retrospective Chart Review Evaluating Concomitant Methotrexate De-escalation Patterns in Patients with Rheumatoid Arthritis Treated with Biologic or Targeted Synthetic DMARDs
- PMID: 38976169
- PMCID: PMC11422300
- DOI: 10.1007/s40744-024-00696-9
A Canadian Retrospective Chart Review Evaluating Concomitant Methotrexate De-escalation Patterns in Patients with Rheumatoid Arthritis Treated with Biologic or Targeted Synthetic DMARDs
Abstract
Introduction: Rheumatoid arthritis (RA) guidelines recommend methotrexate (MTX)-anchored therapy with biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs); however, tolerability issues often lead to non-adherence. Canadian data on MTX tapering and/or withdrawal following b/tsDMARD initiation are minimal. This chart review assessed frequency of MTX tapering or withdrawal following b/tsDMARD initiation and the impact on disease status in Canadian adults with RA.
Methods: Eligible patients had received MTX for ≥ 3 months before b/tsDMARD initiation. The b/tsDMARD was prescribed continuously for ≥ 18 months. Patients taking > 10 mg/day oral prednisone or equivalent were excluded.
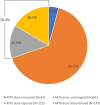
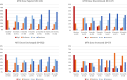
Results: Eight hundred eighty-nine patients (mean baseline MTX dose 19.0 mg/week) prescribed b/tsDMARDs (tumor necrosis factor inhibitor 52.1%, Janus kinase inhibitor 18.3%, interleukin-6 inhibitor [IL-6i] 11.9%, other 17.7%) were evaluated at 22 Canadian centers. Within 2 years of b/tsDMARD initiation, MTX was tapered in 123 (13.8%) patients and discontinued in 147 (16.5%), most commonly due to planned tapering (36.6%) and patient decision (27.2%), respectively, and most commonly with IL-6i use (34.9%). The MTX dose was unchanged for 582 (65.5%) patients and increased for 37 (4.2%). Missing data limit interpretations of MTX dose effects on some secondary endpoints and challenge the assertion that a disease activity measure-based treat-to-target approach is routinely used in Canadian rheumatology practice.
Conclusions: Methotrexate tapering or withdrawal occurred in 30.4% of Canadians with RA within 2 years following b/tsDMARD initiation. Baseline disease activity measures were missing from many medical records. However, for patients with baseline assessments, MTX tapering or discontinuation did not worsen disease activity.
Keywords: Antirheumatic agents; Methotrexate; Rheumatoid arthritis; Therapy.
© 2024. The Author(s).
Conflict of interest statement
Louis Bessette has been a speaker and consultant for Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Lilly, Novartis, Sanofi, Sandoz, Fresenius Kabi, Teva, Organon, and JAMP Pharma; and has received research grants from Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Celgene, Sanofi, Lilly, Novartis, Gilead, and JAMP Pharma. Brandusa Florica has been a speaker for AbbVie, BMS, Fresenius Kabi, Merck, Novartis, Pfizer, Roche, Sandoz, UCB; has been an advisory board advisor for AbbVie, Celltrion Healthcare, Jenssen, Pfizer; and has been a principal investigator for AbbVie, JAMP, Pharma, Jenssen, BMS, and Pfizer. Latha Naik declares no competing interests. Dalton Sholter has consulting relationships as a speaker and a consultant and for research with AbbVie, Amgen, BMS, Celgene, Gilead, JAMP, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, and UCB; and is a Partner with Rheumatology Research Associates, LTD (Phase 2, 3, 4 drug trials in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis). Pierre-André Fournier is an employee of AbbVie Corporation. Tanya Girard is an employee of AbbVie Corporation. Dalinda Liazoghli is an employee of AbbVie Corporation. Philip A. Baer has received consulting fees, speaker fees, or honoraria from AbbVie, Amgen, AstraZeneca, Eli Lilly, Fresenius Kabi, GSK, Innomar, JAMP Pharma, Janssen, McKesson, Novartis/Sandoz, and Pfizer.
Figures
References
-
- Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25. - PubMed
-
- Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77. - PubMed
-
- Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–82. - PubMed
-
- Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;2012(8):1559–82. - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. - PubMed
LinkOut - more resources
Full Text Sources

