Tissue Mechanics and Hedgehog Signaling Crosstalk as a Key Epithelial-Stromal Interplay in Cancer Development
- PMID: 38976559
- PMCID: PMC11425211
- DOI: 10.1002/advs.202400063
Tissue Mechanics and Hedgehog Signaling Crosstalk as a Key Epithelial-Stromal Interplay in Cancer Development
Abstract
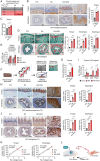
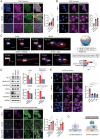
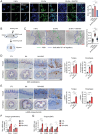
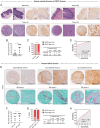
Epithelial-stromal interplay through chemomechanical cues from cells and matrix propels cancer progression. Elevated tissue stiffness in potentially malignant tissues suggests a link between matrix stiffness and enhanced tumor growth. In this study, employing chronic oral/esophageal injury and cancer models, it is demonstrated that epithelial-stromal interplay through matrix stiffness and Hedgehog (Hh) signaling is key in compounding cancer development. Epithelial cells actively interact with fibroblasts, exchanging mechanoresponsive signals during the precancerous stage. Specifically, epithelial cells release Sonic Hh, activating fibroblasts to produce matrix proteins and remodeling enzymes, resulting in tissue stiffening. Subsequently, basal epithelial cells adjacent to the stiffened tissue become proliferative and undergo epithelial-to-mesenchymal transition, acquiring migratory and invasive properties, thereby promoting invasive tumor growth. Notably, transcriptomic programs of oncogenic GLI2, mechano-activated by actin cytoskeletal tension, govern this process, elucidating the crucial role of non-canonical GLI2 activation in orchestrating the proliferation and mesenchymal transition of epithelial cells. Furthermore, pharmacological intervention targeting tissue stiffening proves highly effective in slowing cancer progression. These findings underscore the impact of epithelial-stromal interplay through chemo-mechanical (Hh-stiffness) signaling in cancer development, and suggest that targeting tissue stiffness holds promise as a strategy to disrupt chemo-mechanical feedback, enabling effective cancer treatment.
Keywords: cancer development; chemomechanical cues; epithelial–stroma interplay; hedgehog; tissue stiffness.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Rybinski B., Franco‐Barraza J., Cukierman E., Physiol. Genomics 2014, 46, 223; - PMC - PubMed
- b) Affo S., Yu L. X., Schwabe R. F., Annu. Rev. Pathol. 2017, 12, 153; - PMC - PubMed
- c) Baglieri J., Brenner D. A., Kisseleva T., Int. J. Mol. Sci. 2019, 20, 1723; - PMC - PubMed
- d) Huang C., Iovanna J., Santofimia‐Castaño P., Int. J. Mol. Sci. 2021, 22, 4970; - PMC - PubMed
- e) Oya Y., Hayakawa Y., Koike K., Cancer Sci. 2020, 111, 2696. - PMC - PubMed
-
- a) Mazur A., Holthoff E., Vadali S., Kelly T., Post S. R., PLoS One 2016, 11, e0150287; - PMC - PubMed
- b) Perri R. T., Kay N. E., McCarthy J., Vessella R. L., Jacob H. S., Furcht L. T., Blood 1982, 60, 430; - PubMed
- c) Stahl M., Schupp J., Jäger B., Schmid M., Zissel G., Müller‐Quernheim J., Prasse A., PLoS One 2013, 8, e81382; - PMC - PubMed
- d) Jiang H., Hegde S., DeNardo D. G., Cancer Immunol. Immunother. 2017, 66, 1037. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
