The new science of sleep: From cells to large-scale societies
- PMID: 38976664
- PMCID: PMC11230563
- DOI: 10.1371/journal.pbio.3002684
The new science of sleep: From cells to large-scale societies
Abstract
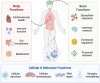
In the past 20 years, more remarkable revelations about sleep and its varied functions have arguably been made than in the previous 200. Building on this swell of recent findings, this essay provides a broad sampling of selected research highlights across genetic, molecular, cellular, and physiological systems within the body, networks within the brain, and large-scale social dynamics. Based on this raft of exciting new discoveries, we have come to realize that sleep, in this moment of its evolution, is very much polyfunctional (rather than monofunctional), yet polyfunctional for reasons we had never previously considered. Moreover, these new polyfunctional insights powerfully reaffirm sleep as a critical biological, and thus health-sustaining, requisite. Indeed, perhaps the only thing more impressive than the unanticipated nature of these newly emerging sleep functions is their striking divergence, from operations of molecular mechanisms inside cells to entire group societal dynamics.
Copyright: © 2024 Sharon et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

