An mRNA vaccine for pancreatic cancer designed by applying in silico immunoinformatics and reverse vaccinology approaches
- PMID: 38976715
- PMCID: PMC11230540
- DOI: 10.1371/journal.pone.0305413
An mRNA vaccine for pancreatic cancer designed by applying in silico immunoinformatics and reverse vaccinology approaches
Abstract
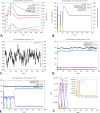
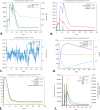
Pancreatic ductal adenocarcinoma is the most prevalent pancreatic cancer, which is considered a significant global health concern. Chemotherapy and surgery are the mainstays of current pancreatic cancer treatments; however, a few cases are suitable for surgery, and most of the cases will experience recurrent episodes. Compared to DNA or peptide vaccines, mRNA vaccines for pancreatic cancer have more promise because of their delivery, enhanced immune responses, and lower proneness to mutation. We constructed an mRNA vaccine by analyzing S100 family proteins, which are all major activators of receptors for advanced glycation end products. We applied immunoinformatic approaches, including physicochemical properties analysis, structural prediction and validation, molecular docking study, in silico cloning, and immune simulations. The designed mRNA vaccine was estimated to have a molecular weight of 165023.50 Da and was highly soluble (grand average of hydropathicity of -0.440). In the structural assessment, the vaccine seemed to be a well-stable and functioning protein (Z score of -8.94). Also, the docking analysis suggested that the vaccine had a high affinity for TLR-2 and TLR-4 receptors. Additionally, the molecular mechanics with generalized Born and surface area solvation analysis of the "Vaccine-TLR-2" (-141.07 kcal/mol) and "Vaccine-TLR-4" (-271.72 kcal/mol) complexes also suggests a strong binding affinity for the receptors. Codon optimization also provided a high expression level with a GC content of 47.04% and a codon adaptation index score 1.0. The appearance of memory B-cells and T-cells was also observed over a while, with an increased level of helper T-cells and immunoglobulins (IgM and IgG). Moreover, the minimum free energy of the mRNA vaccine was predicted at -1760.00 kcal/mol, indicating the stability of the vaccine following its entry, transcription, and expression. This hypothetical vaccine offers a groundbreaking tool for future research and therapeutic development of pancreatic cancer.
Copyright: © 2024 Masum et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

