Mutant androgen receptor induces neurite loss and senescence independently of ARE binding in a neuronal model of SBMA
- PMID: 38976730
- PMCID: PMC11260106
- DOI: 10.1073/pnas.2321408121
Mutant androgen receptor induces neurite loss and senescence independently of ARE binding in a neuronal model of SBMA
Abstract
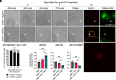
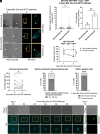
Spinal and bulbar muscular atrophy (SBMA) is a slowly progressing neuromuscular disease caused by a polyglutamine (polyQ)-encoding CAG trinucleotide repeat expansion in the androgen receptor (AR) gene, leading to AR aggregation, lower motor neuron death, and muscle atrophy. AR is a ligand-activated transcription factor that regulates neuronal architecture and promotes axon regeneration; however, whether AR transcriptional functions contribute to disease pathogenesis is not fully understood. Using a differentiated PC12 cell model of SBMA, we identified dysfunction of polyQ-expanded AR in its regulation of neurite growth and maintenance. Specifically, we found that in the presence of androgens, polyQ-expanded AR inhibited neurite outgrowth, induced neurite retraction, and inhibited neurite regrowth. This dysfunction was independent of polyQ-expanded AR transcriptional activity at androgen response elements (ARE). We further showed that the formation of polyQ-expanded AR intranuclear inclusions promoted neurite retraction, which coincided with reduced expression of the neuronal differentiation marker β-III-Tubulin. Finally, we revealed that cell death is not the primary outcome for cells undergoing neurite retraction; rather, these cells become senescent. Our findings reveal that mechanisms independent of AR canonical transcriptional activity underly neurite defects in a cell model of SBMA and identify senescence as a pathway implicated in this pathology. These findings suggest that in the absence of a role for AR canonical transcriptional activity in the SBMA pathologies described here, the development of SBMA therapeutics that preserve this activity may be desirable. This approach may be broadly applicable to other polyglutamine diseases such as Huntington's disease and spinocerebellar ataxias.
Keywords: SBMA; neurite; senescence; transcription.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





References
-
- Kennedy W. R., Alter M., Sung J. H., Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18, 671–680 (1968). - PubMed
-
- Katsuno M., et al. , Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002). - PubMed
-
- Soraru G., et al. , Spinal and bulbar muscular atrophy: Skeletal muscle pathology in male patients and heterozygous females. J. Neurol. Sci. 264, 100–105 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

