Insulin receptor orchestrates kidney antibacterial defenses
- PMID: 38976738
- PMCID: PMC11260129
- DOI: 10.1073/pnas.2400666121
Insulin receptor orchestrates kidney antibacterial defenses
Abstract
Urinary tract infection (UTI) commonly afflicts people with diabetes. This augmented infection risk is partly due to deregulated insulin receptor (IR) signaling in the kidney collecting duct. The collecting duct is composed of intercalated cells (ICs) and principal cells (PCs). Evidence suggests that ICs contribute to UTI defenses. Here, we interrogate how IR deletion in ICs impacts antibacterial defenses against uropathogenic Escherichia coli. We also explore how IR deletion affects immune responses in neighboring PCs with intact IR expression. To accomplish this objective, we profile the transcriptomes of IC and PC populations enriched from kidneys of wild-type and IC-specific IR knock-out mice that have increased UTI susceptibility. Transcriptomic analysis demonstrates that IR deletion suppresses IC-integrated stress responses and innate immune defenses. To define how IR shapes these immune defenses, we employ murine and human kidney cultures. When challenged with bacteria, murine ICs and human kidney cells with deregulated IR signaling cannot engage central components of the integrated stress response-including activating transcriptional factor 4 (ATF4). Silencing ATF4 impairs NFkB activation and promotes infection. In turn, NFkB silencing augments infection and suppresses antimicrobial peptide expression. In diabetic mice and people with diabetes, collecting duct cells show reduced IR expression, impaired integrated stress response engagement, and compromised immunity. Collectively, these translational data illustrate how IR orchestrates collecting duct antibacterial responses and the communication between ICs and PCs.
Keywords: NF-κB; collecting duct; insulin receptor; intercalated cells; pyelonephritis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




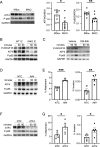
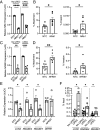



Similar articles
-
Insulin receptor signaling engages bladder urothelial defenses that limit urinary tract infection.Cell Rep. 2024 Apr 23;43(4):114007. doi: 10.1016/j.celrep.2024.114007. Epub 2024 Mar 21. Cell Rep. 2024. PMID: 38517889 Free PMC article.
-
Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses.J Clin Invest. 2018 Dec 3;128(12):5634-5646. doi: 10.1172/JCI98595. Epub 2018 Nov 12. J Clin Invest. 2018. PMID: 30418175 Free PMC article.
-
Acidosis induces antimicrobial peptide expression and resistance to uropathogenic E. coli infection in kidney collecting duct cells via HIF-1α.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F468-F474. doi: 10.1152/ajprenal.00228.2019. Epub 2019 Dec 16. Am J Physiol Renal Physiol. 2020. PMID: 31841391 Free PMC article.
-
Intercalated cell function, kidney innate immunity, and urinary tract infections.Pflugers Arch. 2024 Apr;476(4):565-578. doi: 10.1007/s00424-024-02905-4. Epub 2024 Jan 16. Pflugers Arch. 2024. PMID: 38227050 Review.
-
A role for collecting duct epithelial cells in renal antibacterial defences.Cell Microbiol. 2011 Aug;13(8):1107-13. doi: 10.1111/j.1462-5822.2011.01614.x. Epub 2011 May 25. Cell Microbiol. 2011. PMID: 21615666 Review.
Cited by
-
PTEN modulates urinary tract infection susceptibility and shapes urothelial antibacterial defenses.Life Sci Alliance. 2025 Jul 23;8(10):e202503292. doi: 10.26508/lsa.202503292. Print 2025 Oct. Life Sci Alliance. 2025. PMID: 40701780 Free PMC article.
References
-
- Shah B. R., Hux J. E., Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26, 510–513 (2003). - PubMed
-
- Muller L. M., et al. , Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 41, 281–288 (2005). - PubMed
-
- Kim B., et al. , Diabetes mellitus increases mortality in acute pyelonephritis patients: A population study based on the National Health Insurance Claim Data of South Korea for 2010–2014. Infection 48, 435–443 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- L40 DK130152/DK/NIDDK NIH HHS/United States
- P30CA016058/Ohio State University Comprehensive Cancer Center - Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (The James)
- R50 CA211524/CA/NCI NIH HHS/United States
- R01 DK114035/DK/NIDDK NIH HHS/United States
- L40DK130152/HHS | NIH | Division of Loan Repayment (DLR)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

