Cytochrome oxidase requirements in Bordetella reveal insights into evolution towards life in the mammalian respiratory tract
- PMID: 38976749
- PMCID: PMC11257404
- DOI: 10.1371/journal.ppat.1012084
Cytochrome oxidase requirements in Bordetella reveal insights into evolution towards life in the mammalian respiratory tract
Abstract
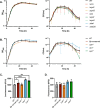
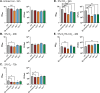
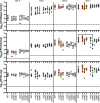
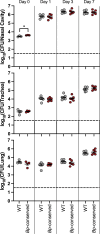
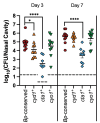
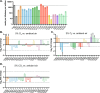
Little is known about oxygen utilization during infection by bacterial respiratory pathogens. The classical Bordetella species, including B. pertussis, the causal agent of human whooping cough, and B. bronchiseptica, which infects nearly all mammals, are obligate aerobes that use only oxygen as the terminal electron acceptor for electron transport-coupled oxidative phosphorylation. B. bronchiseptica, which occupies many niches, has eight distinct cytochrome oxidase-encoding loci, while B. pertussis, which evolved from a B. bronchiseptica-like ancestor but now survives exclusively in and between human respiratory tracts, has only three functional cytochrome oxidase-encoding loci: cydAB1, ctaCDFGE1, and cyoABCD1. To test the hypothesis that the three cytochrome oxidases encoded within the B. pertussis genome represent the minimum number and class of cytochrome oxidase required for respiratory infection, we compared B. bronchiseptica strains lacking one or more of the eight possible cytochrome oxidases in vitro and in vivo. No individual cytochrome oxidase was required for growth in ambient air, and all three of the cytochrome oxidases conserved in B. pertussis were sufficient for growth in ambient air and low oxygen. Using a high-dose, large-volume persistence model and a low-dose, small-volume establishment of infection model, we found that B. bronchiseptica producing only the three B. pertussis-conserved cytochrome oxidases was indistinguishable from the wild-type strain for infection. We also determined that CyoABCD1 is sufficient to cause the same level of bacterial burden in mice as the wild-type strain and is thus the primary cytochrome oxidase required for murine infection, and that CydAB1 and CtaCDFGE1 fulfill auxiliary roles or are important for aspects of infection we have not assessed, such as transmission. Our results shed light on the environment at the surface of the ciliated epithelium, respiration requirements for bacteria that colonize the respiratory tract, and the evolution of virulence in bacterial pathogens.
Copyright: © 2024 McKay et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Nuanced differences in adenylate cyclase toxin production, acylation, and secretion may contribute to the evolution of virulence in Bordetella species.mBio. 2025 Jun 11;16(6):e0108225. doi: 10.1128/mbio.01082-25. Epub 2025 May 19. mBio. 2025. PMID: 40387377 Free PMC article.
-
Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica.Nat Genet. 2003 Sep;35(1):32-40. doi: 10.1038/ng1227. Epub 2003 Aug 10. Nat Genet. 2003. PMID: 12910271
-
Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica.Mol Microbiol. 1996 Dec;22(5):895-908. doi: 10.1046/j.1365-2958.1996.01538.x. Mol Microbiol. 1996. PMID: 8971711
-
Environmental sensing mechanisms in Bordetella.Adv Microb Physiol. 2001;44:141-81. doi: 10.1016/s0065-2911(01)44013-6. Adv Microb Physiol. 2001. PMID: 11407112 Review.
-
Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens.FEMS Immunol Med Microbiol. 2003 Jul 15;37(2-3):121-8. doi: 10.1016/S0928-8244(03)00068-3. FEMS Immunol Med Microbiol. 2003. PMID: 12832115 Review.
Cited by
-
Dioxygen reductase heterogeneity is crucial for robust aerobic growth physiology of Escherichia coli.iScience. 2024 Nov 28;27(12):111498. doi: 10.1016/j.isci.2024.111498. eCollection 2024 Dec 20. iScience. 2024. PMID: 39759019 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

