Molecular surveillance of zoonotic pathogens from wild rodents in the Republic of Korea
- PMID: 38976750
- PMCID: PMC11257403
- DOI: 10.1371/journal.pntd.0012306
Molecular surveillance of zoonotic pathogens from wild rodents in the Republic of Korea
Abstract
Background: Rodents are recognized as major reservoirs of numerous zoonotic pathogens and are involved in the transmission and maintenance of infectious diseases. Furthermore, despite their importance, diseases transmitted by rodents have been neglected. To date, there have been limited epidemiological studies on rodents, and information regarding their involvement in infectious diseases in the Republic of Korea (ROK) is still scarce.
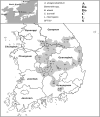
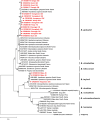
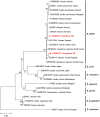
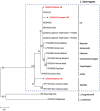
Methodology/principal findings: We investigated rodent-borne pathogens using nested PCR/RT-PCR from 156 rodents including 151 Apodemus agrarius and 5 Rattus norvegicus from 27 regions in eight provinces across the ROK between March 2019 and November 2020. Spleen, kidney, and blood samples were used to detect Anaplasma phagocytophilum, Bartonella spp., Borrelia burgdorferi sensu lato group, Coxiella burnetii, Leptospira interrogans, and severe fever with thrombocytopenia syndrome virus (SFTSV). Of the 156 rodents, 73 (46.8%) were infected with Bartonella spp., 25 (16.0%) with C. burnetii, 24 (15.4%) with L. interrogans, 21 (13.5%) with A. phagocytophilum, 9 (5.8%) with SFTSV, and 5 (3.2%) with Borrelia afzelii. Co-infections with two and three pathogens were detected in 33 (21.1%) and 11 rodents (7.1%), respectively. A. phagocytophilum was detected in all regions, showing a widespread occurrence in the ROK. The infection rates of Bartonella spp. were 83.3% for B. grahamii and 16.7% for B. taylorii.
Conclusions/significance: To the best of our knowledge, this is the first report of C. burnetii and SFTSV infections in rodents in the ROK. This study also provides the first description of various rodent-borne pathogens through an extensive epidemiological survey in the ROK. These results suggest that rodents harbor various pathogens that pose a potential threat to public health in the ROK. Our findings provide useful information on the occurrence and distribution of zoonotic pathogens disseminated among rodents and emphasize the urgent need for rapid diagnosis, prevention, and control strategies for these zoonotic diseases.
Copyright: © 2024 Choi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Prevalence of Orientia tsutsugamushi, Anaplasma phagocytophilum and Leptospira interrogans in striped field mice in Gwangju, Republic of Korea.PLoS One. 2019 Aug 16;14(8):e0215526. doi: 10.1371/journal.pone.0215526. eCollection 2019. PLoS One. 2019. PMID: 31419222 Free PMC article.
-
Highly prevalent bartonellae and other vector-borne pathogens in small mammal species from the Czech Republic and Germany.Parasit Vectors. 2019 Jul 3;12(1):332. doi: 10.1186/s13071-019-3576-7. Parasit Vectors. 2019. PMID: 31269975 Free PMC article.
-
Survey and Phylogenetic Analysis of Rodents and Important Rodent-Borne Zoonotic Pathogens in Gedu, Bhutan.Korean J Parasitol. 2018 Oct;56(5):521-525. doi: 10.3347/kjp.2018.56.5.521. Epub 2018 Oct 31. Korean J Parasitol. 2018. PMID: 30419740 Free PMC article.
-
Neglected vector-borne zoonoses in Europe: Into the wild.Vet Parasitol. 2018 Feb 15;251:17-26. doi: 10.1016/j.vetpar.2017.12.018. Epub 2017 Dec 25. Vet Parasitol. 2018. PMID: 29426471 Review.
-
Sewer-associated rodents in countries with lower human development, a time-bomb for zoonoses?Res Vet Sci. 2025 Jun;188:105614. doi: 10.1016/j.rvsc.2025.105614. Epub 2025 Mar 17. Res Vet Sci. 2025. PMID: 40120388 Review.
Cited by
-
Identification of zoonotic pathogens in zoo animals in the Republic of Korea.Int J Parasitol Parasites Wildl. 2025 Apr 4;27:101067. doi: 10.1016/j.ijppaw.2025.101067. eCollection 2025 Aug. Int J Parasitol Parasites Wildl. 2025. PMID: 40291813 Free PMC article.
References
-
- Sumangali K, Rajapakse R, Rajakaruna R. Urban rodents as potential reservoirs of zoonoses: a parasitic survey in two selected areas in Kandy district. Ceylon J Sci. (Bio Sci.) 2012;41(1):71–7. 10.4038/cjsbs.v41i1.4539. - DOI
-
- Capizzi D, Bertolino S, Mortelliti A. Rating the rat: global patterns and research priorities in impacts and management of rodent pests. Mamm Rev. 2014;44(2):148–62. 10.1111/mam.12019. - DOI
-
- Dalecky A, Bâ K, Piry S, Lippens C, Diagne CA, Kane M, et al. Range expansion of the invasive house mouse Mus musculus domesticus in Senegal, West Africa: a synthesis of trapping data over three decades, 1983–2014. Mamm Rev. 2015;45(3):176–90. 10.1111/mam.12043. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

