Optogenetics in Pancreatic Islets: Actuators and Effects
- PMID: 38976779
- PMCID: PMC11417442
- DOI: 10.2337/db23-1022
Optogenetics in Pancreatic Islets: Actuators and Effects
Abstract
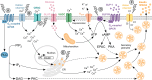
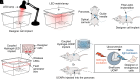
The islets of Langerhans reside within the endocrine pancreas as highly vascularized microorgans that are responsible for the secretion of key hormones, such as insulin and glucagon. Islet function relies on a range of dynamic molecular processes that include Ca2+ waves, hormone pulses, and complex interactions between islet cell types. Dysfunction of these processes results in poor maintenance of blood glucose homeostasis and is a hallmark of diabetes. Recently, the development of optogenetic methods that rely on light-sensitive molecular actuators has allowed perturbation of islet function with near physiological spatiotemporal acuity. These actuators harness natural photoreceptor proteins and their engineered variants to manipulate mouse and human cells that are not normally light-responsive. Until recently, optogenetics in islet biology has primarily focused on controlling hormone production and secretion; however, studies on further aspects of islet function, including paracrine regulation between islet cell types and dynamics within intracellular signaling pathways, are emerging. Here, we discuss the applicability of optogenetics to islets cells and comprehensively review seminal as well as recent work on optogenetic actuators and their effects in islet function and diabetes mellitus.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures

Similar articles
-
Optogenetic Control of Pancreatic Islets.Methods Mol Biol. 2016;1408:107-23. doi: 10.1007/978-1-4939-3512-3_8. Methods Mol Biol. 2016. PMID: 26965119
-
Role of islet peptides in beta cell regulation and type 2 diabetes therapy.Peptides. 2018 Feb;100:212-218. doi: 10.1016/j.peptides.2017.11.014. Peptides. 2018. PMID: 29412821 Review.
-
[Impact of islet alpha cell loss on insulin secretion].Zhonghua Yi Xue Za Zhi. 2002 Oct 25;82(20):1427-31. Zhonghua Yi Xue Za Zhi. 2002. PMID: 12509929 Chinese.
-
Metabolic Stress Impairs Pericyte Response to Optogenetic Stimulation in Pancreatic Islets.Front Endocrinol (Lausanne). 2022 Jun 23;13:918733. doi: 10.3389/fendo.2022.918733. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35813647 Free PMC article.
-
Modulation of the pancreatic islet-stress axis as a novel potential therapeutic target in diabetes mellitus.Vitam Horm. 2014;95:195-222. doi: 10.1016/B978-0-12-800174-5.00008-9. Vitam Horm. 2014. PMID: 24559919 Review.
Cited by
-
A second photoactivatable state of the anion-conducting channelrhodopsin GtACR1 empowers persistent activity.Commun Biol. 2025 Aug 8;8(1):1183. doi: 10.1038/s42003-025-08560-4. Commun Biol. 2025. PMID: 40781123 Free PMC article.
References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005;8:1263–1268 - PubMed
-
- Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron 2002;33:15–22 - PubMed
-
- Sahel J-A, Boulanger-Scemama E, Pagot C, et al. . Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 2021;27:1223–1229 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

