Telescoping a Prenyltransferase and a Diterpene Synthase to Transform Unnatural FPP Derivatives to Diterpenoids
- PMID: 38976793
- PMCID: PMC11267608
- DOI: 10.1021/acs.orglett.4c01670
Telescoping a Prenyltransferase and a Diterpene Synthase to Transform Unnatural FPP Derivatives to Diterpenoids
Abstract
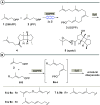
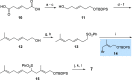
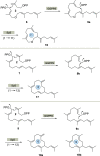
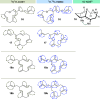
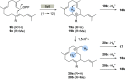
New diterpenoids are accessible from non-natural FPP derivatives as substrates for an enzymatic elongation cyclization cascade using the geranylgeranyl pyrophosphate synthase (GGPPS) from Streptomyces cyaneofuscatus and the spata-13,17-diene synthase (SpS) from Streptomyces xinghaiensis. This approach led to four new biotransformation products including three new cyclododecane cores and a macrocyclic ether. For the first time, a 1,12-terpene cyclization was observed when shifting the central olefinic double bond toward the geminial methyl groups creating a nonconjugated 1,4-diene.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Lynen F.; Eggerer H.; Henning U.; Kessel I. Farnesyl-pyrophosphat und 3-Methyl-Δ3-butenyl-1-pyrophosphat, die biologischen Vorsstufen des Squalens. Angew. Chem. 1958, 70, 738–742. 10.1002/ange.19580702403. - DOI
-
- Ravi B. N.; Wells R. J. A series of new diterpenes from the brown alga Dipholus marginatus (Dictyotaceae). Aust. J. Chem. 1982, 35, 129–144. 10.1071/CH9820129. - DOI
-
- Gerwick W. H.; Fenical W.; van Engen D.; Clardy J. Isolation and Structure of Spatol, a Potent Inhibitor of Cell Replication from the Brown Seaweed Spatoglossum schmittii. J. Am. Chem. Soc. 1980, 102, 7991–7993. 10.1021/ja00547a055. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

