Modeling Ligand Exchange Kinetics in Iridium Complexes Catalyzing SABRE Nuclear Spin Hyperpolarization
- PMID: 38976810
- PMCID: PMC11270526
- DOI: 10.1021/acs.analchem.4c01374
Modeling Ligand Exchange Kinetics in Iridium Complexes Catalyzing SABRE Nuclear Spin Hyperpolarization
Abstract
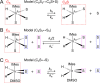
Large signal enhancements can be obtained for NMR analytes using the process of nuclear spin hyperpolarization. Organometallic complexes that bind parahydrogen can themselves become hyperpolarized. Moreover, if parahydrogen and a to-be-hyperpolarized analyte undergo chemical exchange with the organometallic complex it is possible to catalytically sensitize the detection of the analyte via hyperpolarization transfer through spin-spin coupling in this organometallic complex. This process is called Signal Amplification By Reversible Exchange (SABRE). Signal intensity gains of several orders of magnitude can thus be created for various compounds in seconds. The chemical exchange processes play a defining role in controlling the efficiency of SABRE because the lifetime of the complex must match the spin-spin couplings. Here, we show how analyte dissociation rates in the key model substrates pyridine (the simplest six-membered heterocycle), 4-aminopyridine (a drug), and nicotinamide (an essential vitamin biomolecule) can be examined. This is achieved for the most widely employed SABRE motif that is based on IrIMes-derived catalysts by 1H 1D and 2D exchange NMR spectroscopy techniques. Several kinetic models are evaluated for their accuracy and simplicity. By incorporating variable temperature analysis, the data yields key enthalpies and entropies of activation that are critical for understanding the underlying SABRE catalyst properties and subsequently optimizing behavior through rational chemical design. While several studies of chemical exchange in SABRE have been reported, this work also aims to establish a toolkit on how to quantify chemical exchange in SABRE and ensure that data can be compared reliably.
Conflict of interest statement
The authors declare the following competing financial interest(s): Eduard Chekmenev declares a stake of ownership in XeUS Technologies LTD and Vizma Life Sciences. Eduard Chekmenev serves on the Scientific Advisory Board (SAB) of Vizma Life Sciences.
Figures

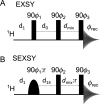
 in this experiment.
in this experiment.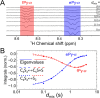
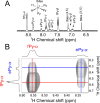
 (Figure 4A). The antiphase line shape of fPy-α at short dmix results in an overall integral close to
zero and does not affect the analysis of the net magnetization exchange.
(Figure 4A). The antiphase line shape of fPy-α at short dmix results in an overall integral close to
zero and does not affect the analysis of the net magnetization exchange.
Similar articles
-
Analysis of chemical exchange in iridium N-heterocyclic carbene complexes using heteronuclear parahydrogen-enhanced NMR.Commun Chem. 2024 Dec 3;7(1):286. doi: 10.1038/s42004-024-01376-z. Commun Chem. 2024. PMID: 39627452 Free PMC article.
-
Theoretical description of hyperpolarization formation in the SABRE-relay method.J Chem Phys. 2020 Oct 28;153(16):164106. doi: 10.1063/5.0023308. J Chem Phys. 2020. PMID: 33138423
-
Analysis of Complex Mixtures by Chemosensing NMR Using para-Hydrogen-Induced Hyperpolarization.Acc Chem Res. 2022 Jul 5;55(13):1832-1844. doi: 10.1021/acs.accounts.1c00796. Epub 2022 Jun 16. Acc Chem Res. 2022. PMID: 35709417 Free PMC article.
-
SABRE: Chemical kinetics and spin dynamics of the formation of hyperpolarization.Prog Nucl Magn Reson Spectrosc. 2019 Oct-Dec;114-115:33-70. doi: 10.1016/j.pnmrs.2019.05.005. Epub 2019 May 25. Prog Nucl Magn Reson Spectrosc. 2019. PMID: 31779885 Review.
-
Synthetic Approaches for 15 N-Labeled Hyperpolarized Heterocyclic Molecular Imaging Agents for 15 N NMR Signal Amplification by Reversible Exchange in Microtesla Magnetic Fields.Chemistry. 2021 Jul 7;27(38):9727-9736. doi: 10.1002/chem.202100212. Epub 2021 May 21. Chemistry. 2021. PMID: 33856077 Free PMC article. Review.
Cited by
-
Analysis of chemical exchange in iridium N-heterocyclic carbene complexes using heteronuclear parahydrogen-enhanced NMR.Commun Chem. 2024 Dec 3;7(1):286. doi: 10.1038/s42004-024-01376-z. Commun Chem. 2024. PMID: 39627452 Free PMC article.
References
-
- Eills J.; Budker D.; Cavagnero S.; Chekmenev E. Y.; Elliott S. J.; Jannin S.; Lesage A.; Matysik J.; Meersmann T.; Prisner T.; Reimer J. A.; Yang H.; Koptyug I. V. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023, 123 (4), 1417–1551. 10.1021/acs.chemrev.2c00534. - DOI - PMC - PubMed
-
- Nelson S. J.; Kurhanewicz J.; Vigneron D. B.; Larson P. E. Z.; Harzstark A. L.; Ferrone M.; van Criekinge M.; Chang J. W.; Bok R.; Park I.; Reed G.; Carvajal L.; Small E. J.; Munster P.; Weinberg V. K.; Ardenkjaer-Larsen J. H.; Chen A. P.; Hurd R. E.; Odegardstuen L.-I.; Robb F. J.; Tropp J.; Murray J. A. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1- 13 C]Pyruvate. Sci. Transl. Med. 2013, 5 (198), 198ra108.10.1126/scitranslmed.3006070. - DOI - PMC - PubMed
-
- Schmidt A. B.; Bowers C. R.; Buckenmaier K.; Chekmenev E. Y.; de Maissin H.; Eills J.; Ellermann F.; Glöggler S.; Gordon J. W.; Knecht S.; Koptyug I. V.; Kuhn J.; Pravdivtsev A. N.; Reineri F.; Theis T.; Them K.; Hövener J.-B. Instrumentation for Hydrogenative Parahydrogen-Based Hyperpolarization Techniques. Anal. Chem. 2022, 94 (1), 479–502. 10.1021/acs.analchem.1c04863. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

