Evolution of Movement Disorders in Patients With CLN2-Batten Disease Treated With Enzyme Replacement Therapy
- PMID: 38976822
- PMCID: PMC11314953
- DOI: 10.1212/WNL.0000000000209615
Evolution of Movement Disorders in Patients With CLN2-Batten Disease Treated With Enzyme Replacement Therapy
Abstract
Objectives: Neuronal ceroid lipofuscinosis type 2 (CLN2-disease) is an inherited childhood-onset neurodegenerative condition, with classical early features of speech delay, epilepsy, myoclonus, ataxia, and motor regression. This study aimed to better characterize the spectrum of movement disorders in CLN2-disease in a cohort of children receiving enzyme replacement therapy (ERT).
Methods: A cohort of 18 children attending a single center for treatment with cerliponase alfa ERT was systematically assessed using a standardized structured history and a double-scored, video-recorded examination using the Unified Batten Disease Rating Scale (UBDRS) and Abnormal Involuntary Movement Scale.
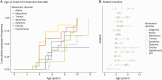
Results: Noncanonical movement disorders are common: while ataxia (89%) and myoclonus (83%) were near-universal, spasticity and dystonia were experienced by over half (61% each), with children having a median of 4 distinct movement disorder phenotypes. This progression was stereotyped with initial ataxia/myoclonus, then hyperkinesia/spasticity, and later hypokinesia. ERT slows progression of movement disorders, as measured by the UBDRS physical subscale, with 1.45 points-per-month progression before diagnosis and 0.44 points-per-month while on treatment (p = 0.019).
Discussion: Movement disorders are a core feature of CLN2-disease and follow a typical pattern of progression which is slowed by ERT. Identifying and treating movement disorders should become standard, especially given increased patient survival.
Conflict of interest statement
R. Spaull has received funding from the Great Ormond Street Hospital Children's Charity and LifeArc. R. Whiteley has received speaker honoraria from BioMarin. J.W. Mink has received research funding from NIH grants P50HD103536 and R01NS060022, and from Neurogene, Amicus, and Theranexus. L. Carr has received speaker honoraria from BioMarin. P. Gissen was an investigator in cerliponase alfa clinical studies for BioMarin, has received consulting fees and grants from BioMarin, has received funding from an NIHR Senior Investigator award (reference NIHR202370), and is a cofounder of/consultant for Bloomsbury Genetic Therapies. M.A. Kurian is a cofounder of/consultant for Bloomsbury Genetic Therapies, has received honoraria from PTC, and has received funding from the MRC (MR/S036784/1), the Great Ormond Street Hospital Children's Charity, and LifeArc. All other authors report no relevant disclosures. Go to
Figures
References
-
- Nickel M, Simonati A, Jacoby D, et al. . Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: an observational cohort study. Lancet Child Adolesc Health. 2018;2(8):582-590. doi:10.1016/S2352-4642(18)30179-2 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
