Crystalloid volume versus catecholamines for management of hemorrhagic shock during esophagectomy: assessment of microcirculatory tissue oxygenation of the gastric conduit in a porcine model using hyperspectral imaging - an experimental study
- PMID: 38976902
- PMCID: PMC11486957
- DOI: 10.1097/JS9.0000000000001849
Crystalloid volume versus catecholamines for management of hemorrhagic shock during esophagectomy: assessment of microcirculatory tissue oxygenation of the gastric conduit in a porcine model using hyperspectral imaging - an experimental study
Abstract
Introduction: Oncologic esophagectomy is a two-cavity procedure with considerable morbidity and mortality. Complex anatomy and the proximity to major vessels constitute a risk for massive intraoperative hemorrhage. Currently, there is no conclusive consensus on the ideal anesthesiologic countermeasure in case of such immense blood loss. The objective of this work was to identify the most promising anesthesiologic management in case of intraoperative hemorrhage with regards to tissue perfusion of the gastric conduit during esophagectomy using hyperspectral imaging.
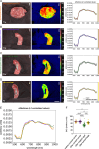
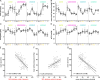
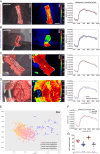
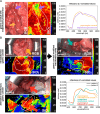
Material and methods: An established live porcine model ( n =32) for esophagectomy was used with gastric conduit formation and simulation of a linear stapled side-to-side esophagogastrostomy. After a standardized procedure of controlled blood loss of about 1 l per pig, the four experimental groups ( n =8 each) differed in anesthesiologic intervention, that is, (I) permissive hypotension, (II) catecholamine therapy using noradrenaline, (III) crystalloid volume supplementation, and (IV) combined crystalloid volume supplementation with noradrenaline therapy. Hyperspectral imaging tissue oxygenation (StO 2 ) of the gastric conduit was evaluated and correlated with systemic perfusion parameters. Measurements were conducted before (T0) and after (T1) laparotomy, after hemorrhage (T2), and 60 min (T3) and 120 min (T4) after anesthesiologic intervention.
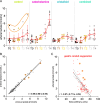
Results: StO 2 values of the gastric conduit showed significantly different results between the four experimental groups, with 63.3% (±7.6%) after permissive hypotension (I), 45.9% (±6.4%) after catecholamine therapy (II), 70.5% (±6.1%) after crystalloid volume supplementation (III), and 69.0% (±3.7%) after combined therapy (IV). StO 2 values correlated strongly with systemic lactate values (r=-0.67; CI -0.77 to -0.54), which is an established prognostic factor.
Conclusion: Crystalloid volume supplementation (III) yields the highest StO 2 values and lowest systemic lactate values and therefore appears to be the superior primary treatment strategy after hemorrhage during esophagectomy with regards to microcirculatory tissue oxygenation of the gastric conduit.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Felix Nickel reports support for courses and travel from Johnson and Johnson, Medtronic, Intuitive Surgical, Cambridge Medical Robotics and KARL STORZ as well as consultancy fees from KARL STORZ. The remaining authors report no conflicts of interest.
Figures



References
-
- Casas MA, Angeramo CA, Bras Harriott C, et al. Surgical outcomes after totally minimally invasive Ivor Lewis esophagectomy. A systematic review and meta-analysis. Eur J Surg Oncol 2022;48:473–481. - PubMed
-
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121–133. - PubMed
-
- Bras Harriott C, Angeramo CA, Casas MA, et al. Open versus hybrid versus totally minimally invasive Ivor Lewis esophagectomy: systematic review and meta-analysis. J Thorac Cardiovasc Surg 2022;164:e233–e254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

