Tumor regression following autologous tumor lysate-loaded dendritic cell vaccination immunotherapy: illustrative case
- PMID: 38976918
- PMCID: PMC11284663
- DOI: 10.3171/CASE24112
Tumor regression following autologous tumor lysate-loaded dendritic cell vaccination immunotherapy: illustrative case
Abstract
Background: Despite years of research, the standard of care (SOC) treatment for grade 4 glioma has remained virtually unchanged for the last 2 decades. Autologous tumor lysate-loaded dendritic cell vaccination (DCVax-L), a novel immunotherapy, has demonstrated a significant survival benefit in a phase 3 trial.
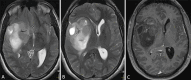
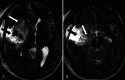
Observations: A 34-year-old male presented with episodes of lightheadedness and was subsequently diagnosed with a large fronto-insulo-temporal tumor, likely to be high grade. He underwent an asleep craniotomy for debulking, with a residual tumor noted in the frontal lobe and amygdala. Tumor histopathology was reported as isocitrate dehydrogenase (IDH) mutant methylated grade 4 astrocytoma. He received SOC treatment, alongside a course of DCVax-L. Surveillance imaging showed cystic transformation followed by a reduction in size of the residual tumor in the frontal lobe; the residual in the amygdala had regressed entirely. The patient remained clinically well and had returned to his preoperative functionality.
Lessons: The authors report a patient with grade 4 astrocytoma who received DCVax-L treatment in addition to SOC adjuvant therapy. The pattern and extent of tumor regression are highly unusual and atypical for what is seen or expected with adjuvant SOC treatment alone. The addition of DCVax-L to SOC opens new avenues in the management of this difficult disease. https://thejns.org/doi/10.3171/CASE24112.
Keywords: DCVax-L; case report; glioblastoma; immunotherapy; treatment; tumor regression.
Figures


Similar articles
-
Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial.JAMA Oncol. 2023 Jan 1;9(1):112-121. doi: 10.1001/jamaoncol.2022.5370. JAMA Oncol. 2023. PMID: 36394838 Free PMC article. Clinical Trial.
-
First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma.J Transl Med. 2018 May 29;16(1):142. doi: 10.1186/s12967-018-1507-6. J Transl Med. 2018. PMID: 29843811 Free PMC article. Clinical Trial.
-
Dendritic cell immunotherapy for solid tumors: evaluation of the DCVax® platform in the treatment of glioblastoma multiforme.CNS Oncol. 2015;4(2):63-9. doi: 10.2217/cns.14.54. CNS Oncol. 2015. PMID: 25768330 Free PMC article. Review.
-
Autologous tumor lysate-loaded dendritic cell vaccination in glioblastoma: What happened to the evidence?Rev Neurol (Paris). 2023 Jun;179(5):502-505. doi: 10.1016/j.neurol.2023.03.014. Epub 2023 Apr 1. Rev Neurol (Paris). 2023. PMID: 37012085 Review.
-
DCVax-L Vaccination in Patients with Glioblastoma: Real Promise or Negative Trial? The Debate Is Open.Cancers (Basel). 2023 Jun 20;15(12):3251. doi: 10.3390/cancers15123251. Cancers (Basel). 2023. PMID: 37370860 Free PMC article.
Cited by
-
Autologous tumor lysate-loaded dendritic cell vaccination in glioblastoma patients: a systematic review of literature.Clin Transl Oncol. 2025 Jul;27(7):2889-2903. doi: 10.1007/s12094-024-03830-9. Epub 2024 Dec 23. Clin Transl Oncol. 2025. PMID: 39714754
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. - PubMed
LinkOut - more resources
Full Text Sources

