An open-label, phase IV randomised controlled trial of two schedules of a four-component meningococcal B vaccine in UK preterm infants
- PMID: 38977298
- PMCID: PMC11503106
- DOI: 10.1136/archdischild-2024-327040
An open-label, phase IV randomised controlled trial of two schedules of a four-component meningococcal B vaccine in UK preterm infants
Abstract
Objective: To compare immunological responses of preterm infants to a four-component meningococcal B vaccine (4CMenB; Bexsero) following a 2+1 vs a 3+1 schedule, and to describe reactogenicity of routine vaccines.
Design: An open-label, phase IV randomised study conducted across six UK sites.
Setting: Neonatal units, postnatal wards, community recruitment following discharge.
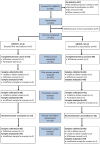
Participants: 129 preterm infants born at a gestation of <35 weeks (64 in group 1 (2+1), 65 in group 2 (3+1)) were included in the analysis. Analysis was completed for postprimary samples from 125 participants (59 in group 1, 66 in group 2) and for postbooster samples from 118 participants (59 in both groups).
Interventions: Infants randomised to 4CMenB according to a 2+1 or a 3+1 schedule, alongside routine vaccines.
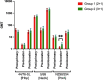
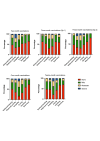
Main outcome measures: Serum bactericidal antibody (SBA) assays performed at 5, 12 and 13 months of age: geometric mean titres (GMTs) and proportions of infants achieving titres ≥4 compared between groups.
Results: There were no significant differences in SBA GMTs between infants receiving a 2+1 compared with a 3+1 schedule following primary or booster vaccination, but a significantly higher proportion of infants had an SBA titre ≥4 against strain NZ98/254 (porin A) at 1 month after primary vaccination using a 3+1 compared with a 2+1 schedule (3+1: 87% (95% CI 76 to 94%), 2+1: 70% (95% CI 56 to 81%), p=0.03).At 12 weeks of age those in the 3+1 group, who received a dose of 4CMenB, had significantly more episodes of fever >38.0°C than those in the 2+1 group who did not (group 2+1: 2% (n=1); 3+1: 14% (n=9); p=0.02).
Conclusions: Both schedules were immunogenic in preterm infants, although a lower response against strain NZ98/254 was seen in the 2+1 schedule; ongoing disease surveillance is important in understanding the clinical significance of this difference.
Trial registration number: NCT03125616.
Keywords: infectious disease medicine; paediatrics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JL and RB perform contract research on behalf of UKHSA for GlaxoSmithKline (GSK), Pfizer and Sanofi. JC works for an institution which conducts meningococcal vaccine research on behalf of GSK; he receives no personal payment or inducement of any kind. MS was an employee of the University of Oxford and Oxford University Hospitals Foundation NHS trust up until September 2022, and in this role acted as an investigator for clinical research studies funded or otherwise supported by the vaccine manufacturers GSK, Janssen, AstraZeneca, Pfizer, Novavax and MCM vaccines. He received no personal financial benefit for this work. As of September 2022, MS has been an employee of Moderna UK and holds equity in this company; however, all study activities and data analysis were completed before this date.
Figures
References
-
- UK Health Security Agency Laboratory confirmed cases of invasive meningococcal infection in England: April to June 2022. https://www.gov.uk/government/publications/meningococcal-disease-laborat... Available.
-
- Davis K, Valente Pinto M, Andrews NJ, et al. Immunogenicity of the UK group B meningococcal vaccine (4CMenB) schedule against groups B and C meningococcal strains (Sched3): outcomes of a multicentre, open-label, randomised controlled trial. Lancet Infect Dis. 2021;21:688–96. doi: 10.1016/S1473-3099(20)30600-9. - DOI - PubMed
-
- Chiappini E, Petrolini C, Sandini E, et al. Update on vaccination of Preterm infants: a systematic review about safety and efficacy/effectiveness. proposal for a position statement by Italian society of pediatric allergology and immunology jointly with the Italian society of neonatology. Expert Rev Vaccines. 2019;18:523–45. doi: 10.1080/14760584.2019.1604230. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
