Lithium-ion battery components are at the nexus of sustainable energy and environmental release of per- and polyfluoroalkyl substances
- PMID: 38977667
- PMCID: PMC11231300
- DOI: 10.1038/s41467-024-49753-5
Lithium-ion battery components are at the nexus of sustainable energy and environmental release of per- and polyfluoroalkyl substances
Abstract
Lithium-ion batteries (LiBs) are used globally as a key component of clean and sustainable energy infrastructure, and emerging LiB technologies have incorporated a class of per- and polyfluoroalkyl substances (PFAS) known as bis-perfluoroalkyl sulfonimides (bis-FASIs). PFAS are recognized internationally as recalcitrant contaminants, a subset of which are known to be mobile and toxic, but little is known about environmental impacts of bis-FASIs released during LiB manufacture, use, and disposal. Here we demonstrate that environmental concentrations proximal to manufacturers, ecotoxicity, and treatability of bis-FASIs are comparable to PFAS such as perfluorooctanoic acid that are now prohibited and highly regulated worldwide, and we confirm the clean energy sector as an unrecognized and potentially growing source of international PFAS release. Results underscore that environmental impacts of clean energy infrastructure merit scrutiny to ensure that reduced CO2 emissions are not achieved at the expense of increasing global releases of persistent organic pollutants.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


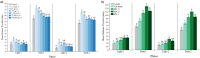

References
-
- Wang Q, Pechy P, Zakeeruddin SM, Exnar I, Grätzel M. Novel electrolytes for Li4Ti5O12-based high power lithium ion batteries with nitrile solvents. J. Power Sour. 2005;146:813–816. doi: 10.1016/j.jpowsour.2005.03.157. - DOI
-
- Kubota K, Tamaki K, Nohira T, Goto T, Hagiwara R. Electrochemical properties of alkali bis(trifluoromethylsulfonyl)amides and their eutectic mixtures. Electrochim. Acta. 2010;55:1113–1119. doi: 10.1016/j.electacta.2009.09.024. - DOI
-
- Rensmo, A. et al. Lithium-ion battery recycling: a source of per- and polyfluoroalkyl substances (PFAS) to the environment? Environ. Sci.: Process. Impacts10.1039/D2EM00511E (2023). - PubMed
-
- Waddell, J. E., Lamanna, W. M., Krause, L. J., Moore, G. G. I. & Hamrock, S. J. Perfluoroalkylsulfonates, sulfonimides, and sulfonyl methides, and electrolytes containing them. US Patent 5,514,493 (1996).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

