Autologous transplant vs. CAR-T therapy in patients with DLBCL treated while in complete remission
- PMID: 38977682
- PMCID: PMC11231252
- DOI: 10.1038/s41408-024-01084-w
Autologous transplant vs. CAR-T therapy in patients with DLBCL treated while in complete remission
Abstract
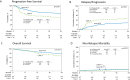
In patients with relapsed DLBCL in complete remission (CR), autologous hematopoietic cell transplantation (auto-HCT) and CAR-T therapy are both effective, but it is unknown which modality provides superior outcomes. We compared the efficacy of auto-HCT vs. CAR-T in patients with DLBCL in a CR. A retrospective observational study comparing auto-HCT (2015-2021) vs. CAR-T (2018-2021) using the Center for International Blood & Marrow Transplant Research registry. Median follow-up was 49.7 months for the auto-HCT and 24.7 months for the CAR-T cohort. Patients ages 18 and 75 with a diagnosis of DLBCL were included if they received auto-HCT (n = 281) or commercial CAR-T (n = 79) while in a CR. Patients undergoing auto-HCT with only one prior therapy line and CAR-T patients with a previous history of auto-HCT treatment were excluded. Endpoints included Progression-free survival (PFS), relapse rate, non-relapse mortality (NRM) and overall survival (OS). In univariate analysis, treatment with auto-HCT was associated with a higher rate of 2-year PFS (66.2% vs. 47.8%; p < 0.001), a lower 2-year cumulative incidence of relapse (27.8% vs. 48% ; p < 0.001), and a superior 2-year OS (78.9% vs. 65.6%; p = 0.037). In patients with early (within 12 months) treatment failure, auto-HCT was associated with a superior 2-year PFS (70.9% vs. 48.3% ; p < 0.001), lower 2-year cumulative incidence of relapse (22.8% vs. 45.9% ; p < 0.001) and trend for higher 2-year OS (82.4% vs. 66.1% ; p = 0.076). In the multivariable analysis, treatment with auto-HCT was associated with a superior PFS (hazard ratio 1.83; p = 0.0011) and lower incidence of relapse (hazard ratio 2.18; p < 0.0001) compared to CAR-T. In patients with relapsed LBCL who achieve a CR, treatment with auto-HCT is associated with improved clinical outcomes compared to CAR-T. These data support the consideration of auto-HCT in select patients with LBCL achieving a CR in the relapsed setting.
© 2024. The Author(s).
Conflict of interest statement
Mazyar Shadman reports Consulting, Advisory Boards, steering committees or data safety monitoring committees: Abbvie, Genentech, AstraZeneca, Janssen, Beigene, Bristol Myers Squibb, Morphosys/Incyte, Kite Pharma, Eli Lilly, Mustang Bio, Fate therapeutics, Nurix, Merck. Research Funding: Mustang Bio, Genentech, AbbVie, Beigene, AstraZeneca, Genmab, Morphosys/Incyte, Vincerx .Stock options: Koi Biotherapeutics. Employment: Bristol Myers Squibb (spouse). Peter Riedell has served as a consultant and/or advisory board member for AbbVie, Novartis, BMS, ADC Therapeutics, Kite/Gilead, Sana Biotechnology, Nektar Therapeutics, Nurix Therapeutics, Intellia Therapeutics, CVS Caremark, Genmab, BeiGene, Janssen, and Pharmacyclics. He has received honoraria from Novartis. Research support from BMS, Kite Pharma, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, AstraZeneca, Genentech, and Tessa Therapeutics. Ajay K. Gopal. Research Funding: Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab. Consultancy/Honoraria: Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi, Scitek. Stock Options: Compliment Corporation. Matthew J Frigault repots consulting and advisory roles for Kite/Gilead, BMS, Novartis, JnJ/Legend. Research funding from Arcellx, Kite/Gilead, Novartis, Incyte. Jonathan Brammer receives research funding from BMS, Incyte and consulting fee from DrenBio, Kymera. Reid Merryman: Advisory board: Genmab, Adaptive Biotechnologies, Bristol Myers Squibb, Abbvie, Inteillia, Epizyme.Consulting: Alphasights, DG Medicine.Institutional research funding: Merck, Bristol Myers Squibb, Genmab, Genentech/Roche. Aleksandr Lazaryan : Consultancy, honoraria, scientific advisory board: Sanofi. Ron Ram: Honoraria: Novartis, Gilead, Takeda, BMS, MSD, Sanofi. Mark Hertzberg: Advisory/honoraria for the following: Takeda, Janssen, Roche, Otsuka, Beigene, Gilead. Farrukh Awan has provided consultancy to: Genentech, Astrazeneca, Abbvie, Janssen, Pharmacyclics, Gilead sciences, Kite pharma,
Figures
References
-
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources