Targeting the transmembrane cytokine co-receptor neuropilin-1 in distal tubules improves renal injury and fibrosis
- PMID: 38977708
- PMCID: PMC11231174
- DOI: 10.1038/s41467-024-50121-6
Targeting the transmembrane cytokine co-receptor neuropilin-1 in distal tubules improves renal injury and fibrosis
Abstract
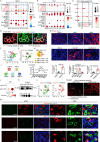
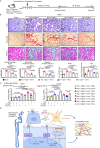
Neuropilin-1 (NRP1), a co-receptor for various cytokines, including TGF-β, has been identified as a potential therapeutic target for fibrosis. However, its role and mechanism in renal fibrosis remains elusive. Here, we show that NRP1 is upregulated in distal tubular (DT) cells of patients with transplant renal insufficiency and mice with renal ischemia-reperfusion (I-R) injury. Knockout of Nrp1 reduces multiple endpoints of renal injury and fibrosis. We find that Nrp1 facilitates the binding of TNF-α to its receptor in DT cells after renal injury. This signaling results in a downregulation of lysine crotonylation of the metabolic enzyme Cox4i1, decreases cellular energetics and exacerbation of renal injury. Furthermore, by single-cell RNA-sequencing we find that Nrp1-positive DT cells secrete collagen and communicate with myofibroblasts, exacerbating acute kidney injury (AKI)-induced renal fibrosis by activating Smad3. Dual genetic deletion of Nrp1 and Tgfbr1 in DT cells better improves renal injury and fibrosis than either single knockout. Together, these results reveal that targeting of NRP1 represents a promising strategy for the treatment of AKI and subsequent chronic kidney disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Yoshioka K, et al. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab. Invest. 1993;68:154–163. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

