Heterogeneity in lung macrophage control of Mycobacterium tuberculosis is modulated by T cells
- PMID: 38977711
- PMCID: PMC11231272
- DOI: 10.1038/s41467-024-48515-7
Heterogeneity in lung macrophage control of Mycobacterium tuberculosis is modulated by T cells
Abstract
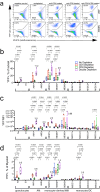
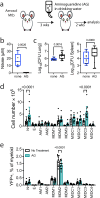
Following Mycobacterium tuberculosis infection, alveolar macrophages are initially infected but ineffectively restrict bacterial replication. The distribution of M. tuberculosis among different cell types in the lung changes with the onset of T cell immunity when the dominant infected cellular niche shifts from alveolar to monocyte-derived macrophages (MDM). We hypothesize that changes in bacterial distribution among different cell types is driven by differences in T cell recognition of infected cells and their subsequent activation of antimicrobial effector mechanisms. We show that CD4 and CD8 T cells efficiently eliminate M. tuberculosis infection in alveolar macrophages, but they have less impact on suppressing infection in MDM, which may be a bacterial niche. Importantly, CD4 T cell responses enhance MDM recruitment to the lung. Thus, the outcome of infection depends on the interaction between the T cell subset and the infected cell; both contribute to the resolution and persistence of the infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Heterogeneity in lung macrophage control of Mycobacterium tuberculosis is determined by T cells.bioRxiv [Preprint]. 2023 Dec 1:2023.11.29.569283. doi: 10.1101/2023.11.29.569283. bioRxiv. 2023. Update in: Nat Commun. 2024 Jul 8;15(1):5710. doi: 10.1038/s41467-024-48515-7. PMID: 38076803 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

