Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer
- PMID: 38977769
- PMCID: PMC11841200
- DOI: 10.1038/s41585-024-00900-z
Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer
Abstract
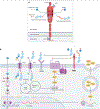
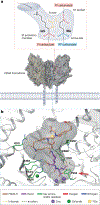
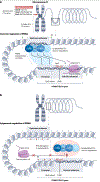
Prostate-specific membrane antigen (PSMA) is an important cell-surface imaging biomarker and therapeutic target in prostate cancer. The PSMA-targeted theranostic 177Lu-PSMA-617 was approved in 2022 for men with PSMA-PET-positive metastatic castration-resistant prostate cancer. However, not all patients respond to PSMA-radioligand therapy, in part owing to the heterogeneity of PSMA expression in the tumour. The PSMA regulatory network is composed of a PSMA transcription complex, an upstream enhancer that loops to the FOLH1 (PSMA) gene promoter, intergenic enhancers and differentially methylated regions. Our understanding of the PSMA regulatory network and the mechanisms underlying PSMA suppression is evolving. Clinically, molecular imaging provides a unique window into PSMA dynamics that occur on therapy and with disease progression, although challenges arise owing to the limited resolution of PET. PSMA regulation and heterogeneity - including intertumoural and inter-patient heterogeneity, temporal changes, lineage dynamics and the tumour microenvironment - affect PSMA theranostics. PSMA response and resistance to radioligand therapy are mediated by a number of potential mechanisms, and complementary biomarkers beyond PSMA are under development. Understanding the biological determinants of cell surface target regulation and heterogeneity can inform precision medicine approaches to PSMA theranostics as well as other emerging therapies.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests: H.B. has served as consultant/advisory board member for Janssen, Astellas, AstraZeneca, Merck, Pfizer, Blue Earth Diagnostics, Amgen, Bayer, Daicchi Sankyo, Sanofi and Novartis, and has received research funding (to institution) from Janssen, AbbVie/Stemcentrx, Bristol Myers Squibb, Circle Pharma, Novartis and Daicchi Sankyo. M.K.B. declares no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

