Blood pressure during long-term cilostazol-based dual antiplatelet therapy after stroke: a post hoc analysis of the CSPS.com trial
- PMID: 38977876
- PMCID: PMC11374707
- DOI: 10.1038/s41440-024-01742-3
Blood pressure during long-term cilostazol-based dual antiplatelet therapy after stroke: a post hoc analysis of the CSPS.com trial
Abstract
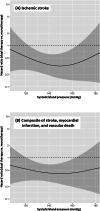
We determined the associations of follow-up blood pressure (BP) after stroke as a time-dependent covariate with the risk of subsequent ischemic stroke, as well as those of BP levels with the difference in the impact of long-term clopidogrel or aspirin monotherapy versus additional cilostazol medication on secondary stroke prevention. In a sub-analysis of a randomized controlled trial (CSPS.com), patients between 8 and 180 days after stroke onset were randomly assigned to receive aspirin or clopidogrel alone, or a combination of cilostazol with aspirin or clopidogrel. The percent changes, differences, and raw values of follow-up BP were examined. The primary efficacy outcome was the first recurrence of ischemic stroke. In a total of 1657 patients (69.5 ± 9.3 years, female 29.1%) with median 1.5-year follow-up, ischemic stroke recurred in 74 patients. The adjusted hazard ratio for ischemic stroke of a 10% systolic BP (SBP) increase from baseline was 1.19 (95% CI 1.03-1.36), that of a 10 mmHg SBP increase was 1.14 (1.03-1.28), and that of SBP as the raw value with the baseline SBP as a fixed (time-independent) covariate was 1.14 (1.00-1.31). Such significant associations were not observed in diastolic BP-derived variables. The estimated adjusted hazard ratio curves for the outcome showed the benefit of dual therapy over a wide SBP range between ≈120 and ≈165 mmHg uniformly. Lower long-term SBP levels after ischemic stroke were associated with a lower risk of subsequent ischemic events. The efficacy of dual antiplatelet therapy including cilostazol for secondary stroke prevention was evident over a wide SBP range.
Keywords: Antiplatelet therapy; Cerebral infarction; Cilostazol; Hypertension; Stroke prevention.
© 2024. The Author(s).
Conflict of interest statement
All of the following are outside the submitted work. Toyoda reports honoraria from Otsuka Pharmaceutical, Daiichi-Sankyo, Bristol-Myers-Squibb, Bayer Yakuhin, and Janssen. Koga reports honoraria from Bayer Yakuhin, Daiichi-Sankyo, Mitsubishi Tanabe Pharma Corporation, and research support from, Daiichi-Sankyo, Nippon Boehringer Ingelheim. Hoshino reports honoraria from Otsuka Pharmaceutical, Bristol-Myers-Squibb and Pfizer. Okada reports honoraria from Daiichi-Sankyo and Pfizer. Sakai reports a research grant from Biomedical Solutions, Medtronic, Terumo and TG Medical; lecturer’s fees from Asahi-Intec, Biomedical Solutions, Daiichi Sankyo, Kaneka, Medtronic, Stryker and Terumo; and membership on the advisory boards for Johnson & Johnson, Medtronic and Terumo. Minematsu reports honoraria from Bayer Yakuhin and Pfizer. None of the other authors have any conflicts of interest to declare.
Figures
Comment in
-
Is it effective to initiate cilostazol-based dual antiplatelet therapy before achieving blood pressure control? Lessons from the CSPS study.Hypertens Res. 2024 Oct;47(10):2939-2941. doi: 10.1038/s41440-024-01813-5. Epub 2024 Aug 8. Hypertens Res. 2024. PMID: 39117951 No abstract available.
References
-
- Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2019;18:539–48. 10.1016/S1474-4422(19)30148-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

