Environmentally dependent interactions shape patterns in gene content across natural microbiomes
- PMID: 38977908
- PMCID: PMC11386527
- DOI: 10.1038/s41564-024-01752-4
Environmentally dependent interactions shape patterns in gene content across natural microbiomes
Erratum in
-
Author Correction: Environmentally dependent interactions shape patterns in gene content across natural microbiomes.Nat Microbiol. 2025 Feb;10(2):599. doi: 10.1038/s41564-024-01899-0. Nat Microbiol. 2025. PMID: 39623069 No abstract available.
Abstract
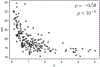
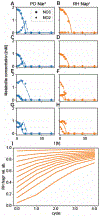
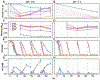
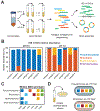
Sequencing surveys of microbial communities in hosts, oceans and soils have revealed ubiquitous patterns linking community composition to environmental conditions. While metabolic capabilities restrict the environments suitable for growth, the influence of ecological interactions on patterns observed in natural microbiomes remains uncertain. Here we use denitrification as a model system to demonstrate how metagenomic patterns in soil microbiomes can emerge from pH-dependent interactions. In an analysis of a global soil sequencing survey, we find that the abundances of two genotypes trade off with pH; nar gene abundances increase while nap abundances decrease with declining pH. We then show that in acidic conditions strains possessing nar fail to grow in isolation but are enriched in the community due to an ecological interaction with nap genotypes. Our study provides a road map for dissecting how associations between environmental variables and gene abundances arise from environmentally modulated community interactions.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures







Update of
-
Global patterns in gene content of soil microbiomes emerge from microbial interactions.bioRxiv [Preprint]. 2023 Nov 15:2023.05.31.542950. doi: 10.1101/2023.05.31.542950. bioRxiv. 2023. Update in: Nat Microbiol. 2024 Aug;9(8):2022-2037. doi: 10.1038/s41564-024-01752-4. PMID: 38014336 Free PMC article. Updated. Preprint.
Similar articles
-
Global patterns in gene content of soil microbiomes emerge from microbial interactions.bioRxiv [Preprint]. 2023 Nov 15:2023.05.31.542950. doi: 10.1101/2023.05.31.542950. bioRxiv. 2023. Update in: Nat Microbiol. 2024 Aug;9(8):2022-2037. doi: 10.1038/s41564-024-01752-4. PMID: 38014336 Free PMC article. Updated. Preprint.
-
Metagenomic analysis reveals distinct patterns of denitrification gene abundance across soil moisture, nitrate gradients.Environ Microbiol. 2019 Apr;21(4):1255-1266. doi: 10.1111/1462-2920.14587. Epub 2019 Mar 21. Environ Microbiol. 2019. PMID: 30851141
-
Genomic fingerprints of the world's soil ecosystems.mSystems. 2024 Jun 18;9(6):e0111223. doi: 10.1128/msystems.01112-23. Epub 2024 May 9. mSystems. 2024. PMID: 38722174 Free PMC article.
-
Revealing the hidden world of soil microbes: Metagenomic insights into plant, bacteria, and fungi interactions for sustainable agriculture and ecosystem restoration.Microbiol Res. 2024 Aug;285:127764. doi: 10.1016/j.micres.2024.127764. Epub 2024 May 15. Microbiol Res. 2024. PMID: 38805978 Review.
-
The known and the unknown in soil microbial ecology.FEMS Microbiol Ecol. 2019 Feb 1;95(2). doi: 10.1093/femsec/fiz005. FEMS Microbiol Ecol. 2019. PMID: 30624643 Review.
Cited by
-
Environmental modulators of algae-bacteria interactions at scale.Cell Syst. 2024 Sep 18;15(9):838-853.e13. doi: 10.1016/j.cels.2024.08.002. Epub 2024 Sep 4. Cell Syst. 2024. PMID: 39236710
-
Dynamic coexistence driven by physiological transitions in microbial communities.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2405527122. doi: 10.1073/pnas.2405527122. Epub 2025 Apr 17. Proc Natl Acad Sci U S A. 2025. PMID: 40244660 Free PMC article.
-
Modeling bacterial interactions uncovers the importance of outliers in the coastal lignin-degrading consortium.Nat Commun. 2025 Jan 14;16(1):639. doi: 10.1038/s41467-025-56012-8. Nat Commun. 2025. PMID: 39809803 Free PMC article.
-
Genetic and species rearrangements in microbial consortia impact biodegradation potential.ISME J. 2025 Jan 2;19(1):wraf014. doi: 10.1093/ismejo/wraf014. ISME J. 2025. PMID: 39861970 Free PMC article.
-
Functional regimes define soil microbiome response to environmental change.Nature. 2025 Aug;644(8078):1028-1038. doi: 10.1038/s41586-025-09264-9. Epub 2025 Jul 16. Nature. 2025. PMID: 40670792 Free PMC article.
References
-
- Wang C.-y. et al. Soil ph is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern china. Annals of Microbiology 69, 1461–1473 (2019).
MeSH terms
Substances
Grants and funding
- EF 2025293/National Science Foundation (NSF)
- R01 GM151538/GM/NIGMS NIH HHS/United States
- R01GM151538/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- DGE 174604/National Science Foundation (NSF)
- 220020499/James S. McDonnell Foundation (McDonnell Foundation)
LinkOut - more resources
Full Text Sources
Research Materials

