Genome-scale metabolic network of human carotid plaque reveals the pivotal role of glutamine/glutamate metabolism in macrophage modulating plaque inflammation and vulnerability
- PMID: 38978031
- PMCID: PMC11232311
- DOI: 10.1186/s12933-024-02339-3
Genome-scale metabolic network of human carotid plaque reveals the pivotal role of glutamine/glutamate metabolism in macrophage modulating plaque inflammation and vulnerability
Abstract
Background: Metabolism is increasingly recognized as a key regulator of the function and phenotype of the primary cellular constituents of the atherosclerotic vascular wall, including endothelial cells, smooth muscle cells, and inflammatory cells. However, a comprehensive analysis of metabolic changes associated with the transition of plaque from a stable to a hemorrhaged phenotype is lacking.
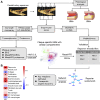
Methods: In this study, we integrated two large mRNA expression and protein abundance datasets (BIKE, n = 126; MaasHPS, n = 43) from human atherosclerotic carotid artery plaque to reconstruct a genome-scale metabolic network (GEM). Next, the GEM findings were linked to metabolomics data from MaasHPS, providing a comprehensive overview of metabolic changes in human plaque.
Results: Our study identified significant changes in lipid, cholesterol, and inositol metabolism, along with altered lysosomal lytic activity and increased inflammatory activity, in unstable plaques with intraplaque hemorrhage (IPH+) compared to non-hemorrhaged (IPH-) plaques. Moreover, topological analysis of this network model revealed that the conversion of glutamine to glutamate and their flux between the cytoplasm and mitochondria were notably compromised in hemorrhaged plaques, with a significant reduction in overall glutamate levels in IPH+ plaques. Additionally, reduced glutamate availability was associated with an increased presence of macrophages and a pro-inflammatory phenotype in IPH+ plaques, suggesting an inflammation-prone microenvironment.
Conclusions: This study is the first to establish a robust and comprehensive GEM for atherosclerotic plaque, providing a valuable resource for understanding plaque metabolism. The utility of this GEM was illustrated by its ability to reliably predict dysregulation in the cholesterol hydroxylation, inositol metabolism, and the glutamine/glutamate pathway in rupture-prone hemorrhaged plaques, a finding that may pave the way to new diagnostic or therapeutic measures.
Keywords: Atherosclerosis; Genome-scale metabolic network; Macrophage; Metabolomics; Plaque rupture.
© 2024. The Author(s).
Conflict of interest statement
Peter Juhasz is employed by PJConsulting. The other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

