CDCA5 accelerates progression of breast cancer by promoting the binding of E2F1 and FOXM1
- PMID: 38978058
- PMCID: PMC11232132
- DOI: 10.1186/s12967-024-05443-w
CDCA5 accelerates progression of breast cancer by promoting the binding of E2F1 and FOXM1
Abstract
Background: Breast cancer is one of the most common malignant tumors in women. Cell division cycle associated 5 (CDCA5), a master regulator of sister chromatid cohesion, was reported to be upregulated in several types of cancer. Here, the function and regulation mechanism of CDCA5 in breast cancer were explored.
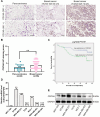
Methods: CDCA5 expression was identified through immunohistochemistry staining in breast cancer specimens. The correlation between CDCA5 expression with clinicopathological features and prognosis of breast cancer patients was analyzed using a tissue microarray. CDCA5 function in breast cancer was explored in CDCA5-overexpressed/knockdown cells and mice models. Co-IP, ChIP and dual-luciferase reporter assay assays were performed to clarify underlying molecular mechanisms.
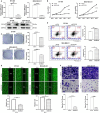
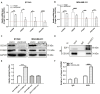
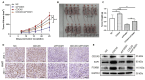
Results: We found that CDCA5 was expressed at a higher level in breast cancer tissues and cell lines, and overexpression of CDCA5 was significantly associated with poor prognosis of patients with breast cancer. Moreover, CDCA5 knockdown significantly suppressed the proliferation and migration, while promoted apoptosis in vitro. Mechanistically, we revealed that CDCA5 played an important role in promoting the binding of E2F transcription factor 1 (E2F1) to the forkhead box M1 (FOXM1) promoter. Furthermore, the data of in vitro and in vivo revealed that depletion of FOXM1 alleviated the effect of CDCA5 overexpression on breast cancer. Additionally, we revealed that the Wnt/β-catenin signaling pathway was required for CDCA5 induced progression of breast cancer.
Conclusions: We suggested that CDCA5 promoted progression of breast cancer via CDCA5/FOXM1/Wnt axis, CDCA5 might serve as a novel therapeutic target for breast cancer treatment.
Keywords: Breast cancer; CDCA5; FOXM1; Wnt/β-catenin signaling.
© 2024. The Author(s).
Conflict of interest statement
Na Shen and Xiangwang Zhao are co-correspondence authors for this study. The authors declare no conflicts of interest in this research.
These authors declare that they have no conflict of interests.
Figures

Similar articles
-
E2F1-Dependent CDCA5 overexpression drives cervical cancer progression and correlates with poor prognosis.J Mol Histol. 2025 Feb 5;56(2):80. doi: 10.1007/s10735-025-10356-z. J Mol Histol. 2025. PMID: 39907709
-
The critical role of dysregulated Hh-FOXM1-TPX2 signaling in human hepatocellular carcinoma cell proliferation.Cell Commun Signal. 2020 Jul 28;18(1):116. doi: 10.1186/s12964-020-00628-4. Cell Commun Signal. 2020. PMID: 32723329 Free PMC article.
-
Overexpression of CDCA5, KIF4A, TPX2, and FOXM1 Coregulated Cell Cycle and Promoted Hepatocellular Carcinoma Development.J Comput Biol. 2020 Jun;27(6):965-974. doi: 10.1089/cmb.2019.0254. Epub 2019 Oct 9. J Comput Biol. 2020. PMID: 31593490
-
FOXM1 transcriptional regulation.Biol Cell. 2024 Sep;116(9):e2400012. doi: 10.1111/boc.202400012. Epub 2024 Jul 4. Biol Cell. 2024. PMID: 38963053 Review.
-
Role of cell division cycle-associated proteins in regulating cell cycle and promoting tumor progression.Biochim Biophys Acta Rev Cancer. 2024 Sep;1879(5):189147. doi: 10.1016/j.bbcan.2024.189147. Epub 2024 Jun 30. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38955314 Review.
Cited by
-
The E2F1/SBK1 axis activates the Notch signaling pathway to accelerate the malignant progression of breast cancer.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 27. doi: 10.1007/s00210-025-04251-3. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40423801
-
The acidic microenvironment promotes pancreatic cancer progression via the lncRNA-LOC100507424/E2F1/FOXM1 axis.BMC Cancer. 2025 Apr 10;25(1):655. doi: 10.1186/s12885-025-14073-4. BMC Cancer. 2025. PMID: 40211195 Free PMC article.
-
E2F1-Dependent CDCA5 overexpression drives cervical cancer progression and correlates with poor prognosis.J Mol Histol. 2025 Feb 5;56(2):80. doi: 10.1007/s10735-025-10356-z. J Mol Histol. 2025. PMID: 39907709
References
-
- Emens LA. Immunotherapy in Triple-negative breast Cancer. Cancer J 2021, 27:59–66. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

