This is a preprint.
B cell-mediated antigen presentation promotes adverse cardiac remodeling in chronic heart failure
- PMID: 38978561
- PMCID: PMC11230502
- DOI: 10.21203/rs.3.rs-4536350/v1
B cell-mediated antigen presentation promotes adverse cardiac remodeling in chronic heart failure
Abstract
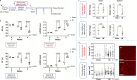
Cardiovascular disease remains the leading cause of death worldwide. A primary driver of cardiovascular mortality is ischemic heart failure, a form of cardiac dysfunction that can develop in patients who survive myocardial infarction. Acute cardiac damage triggers robust changes in the spleen with rapid migration of immune cells from the spleen to the heart. Activating this "cardio-splenic" axis contributes to progressive cardiac dysfunction. The cardio-splenic axis has, therefore, been identified as a promising therapeutic target to prevent or treat heart failure. However, our understanding of the precise mechanisms by which specific immune cells contribute to adverse cardiac remodeling within the cardio-splenic axis remains limited. Here, we show that splenic B cells contribute to the development of heart failure via MHC II-mediated antigen presentation. We found that the adoptive transfer of splenic B cells from mice with ischemic heart failure promoted adverse cardiac remodeling and splenic inflammatory changes in naïve recipient mice. Based on single-cell RNA sequencing analysis of splenic B cells from mice with ischemic heart failure, we hypothesized that B cells contributed to adverse cardiac remodeling through antigen presentation by MHC II molecules. This mechanism was confirmed using transgenic mice with B cell-specific MHC II deletion, and by analyzing circulating B cells from humans who experienced myocardial infarction. Our results broaden our understanding of B lymphocyte biology, reshape current models of immune activation in response to myocardial injury, and point towards MHC II-mediated signaling in B cells as a novel and specific therapeutic target in chronic heart failure.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

