The roles and mechanisms of the NF-κB signaling pathway in tendon disorders
- PMID: 38978635
- PMCID: PMC11228182
- DOI: 10.3389/fvets.2024.1382239
The roles and mechanisms of the NF-κB signaling pathway in tendon disorders
Abstract
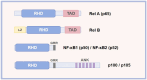
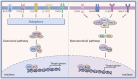
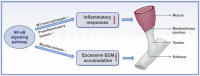
Both acute and chronic tendon injuries are the most frequently occurring musculoskeletal diseases in human and veterinary medicine, with a limited repertoire of successful and evidenced-based therapeutic strategies. Inflammation has been suggested as a key driver for the formation of scar and adhesion tissue following tendon acute injury, as well as pathological alternations of degenerative tendinopathy. However, prior efforts to completely block this inflammatory process have yet to be largely successful. Recent investigations have indicated that a more precise targeted approach for modulating inflammation is critical to improve outcomes. The nuclear factor-kappaB (NF-κB) is a typical proinflammatory signal transduction pathway identified as a key factor leading to tendon disorders. Therefore, a comprehensive understanding of the mechanism or regulation of NF-κB in tendon disorders will aid in developing targeted therapeutic strategies for human and veterinary tendon disorders. In this review, we discuss what is currently known about molecular components and structures of basal NF-κB proteins and two activation pathways: the canonical activation pathway and the non-canonical activation pathway. Furthermore, we summarize the underlying mechanisms of the NF-κB signaling pathway in fibrosis and adhesion after acute tendon injury, as well as pathological changes of degenerative tendinopathy in all species and highlight the effect of targeting this signaling pathway in tendon disorders. However, to gain a comprehensive understanding of its mechanisms underlying tendon disorders, further investigations are required. In the future, extensive scientific examinations are warranted to full characterize the NF-κB, the exact mechanisms of action, and translate findings into clinical human and veterinary practice.
Keywords: NF-κB signaling pathway; tendon adhesion tendinopathy; tendon injury; tendon repair; tendon scar healing.
Copyright © 2024 Li, Li, Luo, Zhang, Feng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Targeting the NF-κB signaling pathway in chronic tendon disease.Sci Transl Med. 2019 Feb 27;11(481):eaav4319. doi: 10.1126/scitranslmed.aav4319. Sci Transl Med. 2019. PMID: 30814338 Free PMC article.
-
NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival.Sci Signal. 2020 Nov 17;13(658):eabb7209. doi: 10.1126/scisignal.abb7209. Sci Signal. 2020. PMID: 33203721 Free PMC article.
-
Inflammation-related signaling pathways in tendinopathy.Open Life Sci. 2023 Sep 20;18(1):20220729. doi: 10.1515/biol-2022-0729. eCollection 2023. Open Life Sci. 2023. PMID: 37744452 Free PMC article. Review.
-
NF-κB signaling in inflammation and cancer.MedComm (2020). 2021 Dec 16;2(4):618-653. doi: 10.1002/mco2.104. eCollection 2021 Dec. MedComm (2020). 2021. PMID: 34977871 Free PMC article. Review.
-
Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy-A Target for Intervention.Int J Mol Sci. 2022 Mar 25;23(7):3571. doi: 10.3390/ijms23073571. Int J Mol Sci. 2022. PMID: 35408931 Free PMC article. Review.
Cited by
-
Insulin-like growth factor-1 (IGF-1) empowering tendon regenerative therapies.Front Bioeng Biotechnol. 2025 Mar 27;13:1492811. doi: 10.3389/fbioe.2025.1492811. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40213631 Free PMC article. Review.
-
Extraction, detection, bioactivity, and product development of luteolin: A review.Heliyon. 2024 Dec 7;10(24):e41068. doi: 10.1016/j.heliyon.2024.e41068. eCollection 2024 Dec 30. Heliyon. 2024. PMID: 39759280 Free PMC article. Review.
-
Branched-Chain Amino Acids and Inflammation Management in Endurance Sports: Molecular Mechanisms and Practical Implications.Nutrients. 2025 Apr 12;17(8):1335. doi: 10.3390/nu17081335. Nutrients. 2025. PMID: 40284200 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

