Sorafenib maintenance in FLT3-ITD mutated AML after allogeneic HCT: a real-world, single-center experience
- PMID: 38978738
- PMCID: PMC11228150
- DOI: 10.3389/fonc.2024.1391743
Sorafenib maintenance in FLT3-ITD mutated AML after allogeneic HCT: a real-world, single-center experience
Abstract
Despite allogeneic hematopoietic stem cell transplant (allo-HCT) and the development of novel FLT3 inhibitors in both induction (midostaurin) and in the relapsed/refractory setting (gilteritinib), FLT3-ITD mutated leukemia (FLT3-ITD+ AML) still represents a challenge for modern hematology. Sorafenib is, to this date, the only inhibitor that demonstrated efficacy in improving both progression-free and overall survival as post-HCT maintenance therapy, even if its use in this setting has not been approved so far by regulatory agencies. The aim of our study was to evaluate the feasibility, safety, and efficacy of sorafenib maintenance in preventing early relapse in FLT3-ITD+ AML after HCT in a single-center experience. We analyzed 26 consecutive patients who received post-HCT 2-year maintenance with sorafenib at our center between 2017 and 2023. The median time from HCT to sorafenib start was 130 days, and the median dosage was 200 mg per day. Two (8%) and three (12%) patients discontinued maintenance due to toxicity and disease relapse, respectively. Eight (31%) patients terminated the 2-year maintenance and stopped sorafenib, while 13 patients are still under treatment. Overall, 21/26 patients (81%) are alive and in stable complete remission as outlined by a 2-year disease-free survival of 83.61%. No major long-term toxicity was reported at the last follow-up. Our real-world experience supports the use of sorafenib as a feasible and effective therapeutic option in post-HCT maintenance for FLT3-ITD+ AML.
Keywords: FLT3 ITD; acute myeloid leukemia; allogeneic stem cell transplantation; maintenance; sorafenib.
Copyright © 2024 Diral, Furnari, Bruno, Greco, Clerici, Marktel, Farina, Mastaglio, Vago, Piemontese, Peccatori, Corti, Bernardi, Ciceri and Lupo-Stanghellini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer CS declared a past co-authorship with the author FC to the handling editor.
Figures
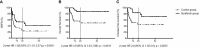
References
-
- Erba HP, Montesinos P, Kim HJ, Patkowska E, Vrhovac R, Žák P, et al. . Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1571–83. doi: 10.1016/S0140-6736(23)00464-6 - DOI - PubMed
-
- Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. . Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. (2020) 38:2993–3002. doi: 10.1200/JCO.19.03345 - DOI - PubMed
-
- Xuan L, Wang Y, Yang K, Shao R, Huang F, Fan Z, et al. . Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. (2023) 10:e600–11. doi: 10.1016/S2352-3026(23)00117-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

