This is a preprint.
The Alzheimer's disease gene SORL1 regulates lysosome function in human microglia
- PMID: 38979155
- PMCID: PMC11230436
- DOI: 10.1101/2024.06.25.600648
The Alzheimer's disease gene SORL1 regulates lysosome function in human microglia
Update in
-
The Alzheimer's Disease Gene SORL1 Regulates Lysosome Function in Human Microglia.Glia. 2025 Jul;73(7):1329-1348. doi: 10.1002/glia.70009. Epub 2025 Apr 4. Glia. 2025. PMID: 40183375 Free PMC article.
Abstract
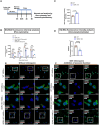
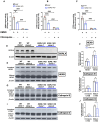
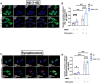
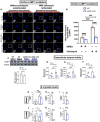
The SORL1 gene encodes the sortilin related receptor protein SORLA, a sorting receptor that regulates endo-lysosomal trafficking of various substrates. Loss of function variants in SORL1 are causative for Alzheimer's disease (AD) and decreased expression of SORLA has been repeatedly observed in human AD brains. SORL1 is highly expressed in the central nervous system, including in microglia, the tissue resident immune cells of the brain. Loss of SORLA leads to enlarged lysosomes in hiPSC-derived microglia like cells (hMGLs). However, how SORLA deficiency contributes to lysosomal dysfunction in microglia and how this contributes to AD pathogenesis is not known. In this study, we show that loss of SORLA results in decreased lysosomal degradation and lysosomal enzyme activity due to altered trafficking of lysosomal enzymes in hMGLs. Phagocytic uptake of fibrillar amyloid beta 1-42 and synaptosomes is increased in SORLA deficient hMGLs, but due to reduced lysosomal degradation, these substrates aberrantly accumulate in lysosomes. An alternative mechanism of lysosome clearance, lysosomal exocytosis, is also impaired in SORL1 deficient microglia, which may contribute to an altered immune response. Overall, these data suggest that SORLA has an important role in proper trafficking of lysosomal hydrolases in hMGLs, which is critical for microglial function. This further substantiates the microglial endo-lysosomal network as a potential novel pathway through which SORL1 may increase AD risk and contribute to development of AD. Additionally, our findings may inform development of novel lysosome and microglia associated drug targets for AD.
Keywords: Alzheimer’s disease; SORL1; hiPSC-derived microglia; lysosomes; phagocytosis.
Figures



References
-
- Armstrong A., Mattsson N., Appelqvist H., Janefjord C., Sandin L., Agholme L., Olsson B., Svensson S., Blennow K., Zetterberg H., & Kågedal K. (2014). Lysosomal network proteins as potential novel CSF biomarkers for Alzheimer’s disease. Neuromolecular Med, 16(1), 150–160. 10.1007/s12017-013-8269-3 - DOI - PMC - PubMed
-
- Baranski T. J., Faust P. L., & Kornfeld S. (1990). Generation of a lysosomal enzyme targeting signal in the secretory protein pepsinogen. Cell, 63(2), 281–291. - PubMed
-
- Bissig C., Hurbain I., Raposo G., & van Niel G. (2017). PIKfyve activity regulates reformation of terminal storage lysosomes from endolysosomes. Traffic, 18(11), 747–757. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
