This is a preprint.
MICOS Complex Loss Governs Age-Associated Murine Mitochondrial Architecture and Metabolism in the Liver, While Sam50 Dictates Diet Changes
- PMID: 38979162
- PMCID: PMC11230271
- DOI: 10.1101/2024.06.20.599846
MICOS Complex Loss Governs Age-Associated Murine Mitochondrial Architecture and Metabolism in the Liver, While Sam50 Dictates Diet Changes
Abstract
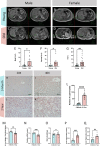
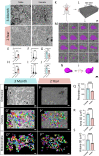
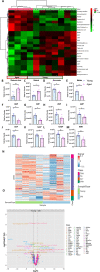
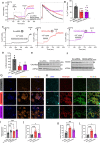
The liver, the largest internal organ and a metabolic hub, undergoes significant declines due to aging, affecting mitochondrial function and increasing the risk of systemic liver diseases. How the mitochondrial three-dimensional (3D) structure changes in the liver across aging, and the biological mechanisms regulating such changes confers remain unclear. In this study, we employed Serial Block Face-Scanning Electron Microscopy (SBF-SEM) to achieve high-resolution 3D reconstructions of murine liver mitochondria to observe diverse phenotypes and structural alterations that occur with age, marked by a reduction in size and complexity. We also show concomitant metabolomic and lipidomic changes in aged samples. Aged human samples reflected altered disease risk. To find potential regulators of this change, we examined the Mitochondrial Contact Site and Cristae Organizing System (MICOS) complex, which plays a crucial role in maintaining mitochondrial architecture. We observe that the MICOS complex is lost during aging, but not Sam50. Sam50 is a component of the sorting and assembly machinery (SAM) complex that acts in tandem with the MICOS complex to modulate cristae morphology. In murine models subjected to a high-fat diet, there is a marked depletion of the mitochondrial protein SAM50. This reduction in Sam50 expression may heighten the susceptibility to liver disease, as our human biobank studies corroborate that Sam50 plays a genetically regulated role in the predisposition to multiple liver diseases. We further show that changes in mitochondrial calcium dysregulation and oxidative stress accompany the disruption of the MICOS complex. Together, we establish that a decrease in mitochondrial complexity and dysregulated metabolism occur with murine liver aging. While these changes are partially be regulated by age-related loss of the MICOS complex, the confluence of a murine high-fat diet can also cause loss of Sam50, which contributes to liver diseases. In summary, our study reveals potential regulators that affect age-related changes in mitochondrial structure and metabolism, which can be targeted in future therapeutic techniques.
Keywords: 3D Structure; Aging; Liver Disease; MICOS Complex; Metabolism; Mitochondria.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare that they have no conflict of interest. All authors have no competing interests.
Figures


References
Publication types
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 DK133698/DK/NIDDK NIH HHS/United States
- R01 HD090061/HD/NICHD NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- S10 RR025141/RR/NCRR NIH HHS/United States
- R01 HL147818/HL/NHLBI NIH HHS/United States
- R01 DA057204/DA/NIDA NIH HHS/United States
- R25 HL106365/HL/NHLBI NIH HHS/United States
- R01 HL144941/HL/NHLBI NIH HHS/United States
- R01 HD074711/HD/NICHD NIH HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- U01 HG006378/HG/NHGRI NIH HHS/United States
- S10 OD017985/OD/NIH HHS/United States
- R01 AI136740/AI/NIAID NIH HHS/United States
- R01 AI170228/AI/NIAID NIH HHS/United States
- R21 DK119879/DK/NIDDK NIH HHS/United States
- I01 BX005352/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- RC2 GM092618/GM/NIGMS NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- R03 HL155041/HL/NHLBI NIH HHS/United States
- K01 AG062757/AG/NIA NIH HHS/United States
- R00 AG065445/AG/NIA NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- R01 NS032830/NS/NINDS NIH HHS/United States
- U01 HG004798/HG/NHGRI NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- R00 DK120876/DK/NIDDK NIH HHS/United States
- T32 DK007563/DK/NIDDK NIH HHS/United States
- R25 AI164610/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
